Novel strategy for treatment of viral central nervous system infection by using a cell-permeating inhibitor of c-Jun N-terminal kinase - PubMed (original) (raw)
Novel strategy for treatment of viral central nervous system infection by using a cell-permeating inhibitor of c-Jun N-terminal kinase
J David Beckham et al. J Virol. 2007 Jul.
Abstract
Viral encephalitis is a major cause of morbidity and mortality worldwide, yet there is no proven efficacious therapy for most viral infections of the central nervous system (CNS). Many of the viruses that cause encephalitis induce apoptosis and activate c-Jun N-terminal kinase (JNK) following infection. We have previously shown that reovirus infection of epithelial cell lines activates JNK-dependent apoptosis. We now show that reovirus infection resulted in activation of JNK and caspase-3 in the CNS. Treatment of reovirus-infected mice with a cell-permeating peptide that competitively inhibits JNK activity resulted in significantly prolonged survival of intracerebrally infected mice following an otherwise lethal challenge with T3D (100 x 50% lethal dose). Protection correlated with reduced CNS injury, reduced neuronal apoptosis, and reduced c-Jun activation without altering the viral titer or viral antigen distribution. Given the efficacy of the inhibitor in protecting mice from viral encephalitis, JNK inhibition represents a promising and novel treatment strategy for viral encephalitis.
Figures
FIG. 1.
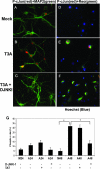
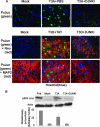
Reovirus-induced activation of pS73 c-Jun in primary cortical neuronal culture is inhibited with D-JNKI-1. Representative photographs are shown from 48 h postinfection. Primary MCCs were mock infected, T3A infected, or treated with D-JNKI-1 prior to T3A infection. (A to C) Primary MCCs were colabeled with antibodies to pS73 c-Jun (red) and neuron-specific antibodies to MAP2 (green). (D to F) Primary MCCs were colabeled with antibodies to pS73 c-Jun activation (red) and antibodies to σ3 reovirus antigen (green). (G) Graphic display of the percentage of primary MCCs that stained positive for pS73 c-Jun. Blinded cell counts from three replicates are shown of mock-infected or T3A-infected primary MCCs treated with D-JNKI-1, TAT control, or vehicle (PBS). Vertical bars indicate standard errors of the means. *, P ≤ 0.05. Original magnification of images shown in panel A, ×400.
FIG. 2.
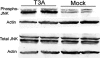
T3A-induced JNK phosphorylation in brains of neonatal mice. Two-day-old Swiss Webster mice were mock or T3A infected and sacrificed when moribund. Tissue was processed for whole-brain lysates and subjected to immunoblotting for evidence of phosphorylated JNK and total JNK. Each band represents an individual.
FIG. 3.
T3A-induced activation of c-Jun in brains of neonatal mice. Two-day-old Swiss Webster mice were mock or T3A infected and sacrificed when moribund. (A) Histological tissue sections from T3A-infected mice were evaluated using fluorescence immunohistochemistry for pS73 c-Jun (green) in the cingulate cortex, hippocampus, and thalamus. Images (original magnification, ×400) provide representative observations of the 12 animals sampled per group. (B) Whole-brain lysate samples were evaluated for evidence of activated (pS63) c-Jun. Each band represents an individual. Images are representative of eight animals sampled per group.
FIG. 4.
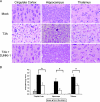
D-JNKI-1 treatment protects from T3A-induced CNS injury. Mice were treated with D-JNKI-1 or vehicle control, followed by viral challenge with T3A. (A) Representative brain tissue samples were stained with hematoxylin and eosin in order to evaluate for evidence of histological damage. Samples represent four replicates of the experiment consisting of six mice per treatment group per replicate. Original magnification of images shown in panel A,×200. (B) Blind neuropathology scoring of histologic sections show significantly decreased injury in the D-JNKI-1-treated mice compared to untreated, T3A-infected mice in areas typical of reovirus histologic injury: the cingulate cortex (*, P = 0.005), hippocampus (*, P = 0.02), and thalamus (*, P = 0.006). Histopathology scoring: 0, no lesions; 1, <10% of total section shows CNS injury; 2, 10 to 40%; 3, 40 to 75%; 4, >75%.
FIG. 5.
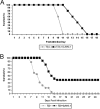
D-JNKI-1 treatment results in significantly prolonged survival for both T3A- and T3D-infected mice. (A) Mice were treated with D-JNKI-1, challenged with T3A 6 h later, and sacrificed when moribund. T3A-infected, untreated mice had an MDD of 10.6 (standard deviation [SD], ±0.6), and T3A-infected, D-JNKI-1 treated mice had an MDD of 14 (SD, ±1.9; P = 0.006). (B) Mice were challenged with T3D and treated 24 h later with vehicle control, TAT control peptide, or D-JNKI-1. Survival studies revealed 38% long-term survival in the T3D-infected, D-JNKI-1-treated group. Of the individuals that died, the MDD was prolonged for T3D-infected, D-JNKI-1-treated mice compared to T3D-infected, untreated mice (13.2; SD, ±1.42; and 9.8; SD, ±2.3 respectively; P < 0.00001). Survival of TAT peptide-treated T3D-infected mice did not significantly differ from that of vehicle control, T3D-infected mice (data not shown).
FIG. 6.
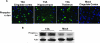
D-JNKI-1 treatment of T3A- or T3D-infected mice decreases activation of c-Jun in vivo. (A) Representative immunohistochemical staining of brain sections of mock-, T3A-, or T3D-infected mice treated with vehicle (PBS), TAT control peptide, or D-JNKI-1 (DJNKI). Successive sections were stained with antibodies to pS73 c-Jun (PcJun) (green) and colabeled with either antibody to reovirus σ3 protein (red) or antibody to MAP2 neuron-specific antigen (red). Images are provided from the cingulate cortex (T3A) or the hippocampus (T3D); original magnification, ×400. (B) Representative immunoblots of whole-brain lysate are shown following mock or T3A infection and treatment with vehicle (PBS) or D-JNKI-1. Blots were probed for pS63 c-Jun and actin. Densitometry studies represent values obtained from individual blots from three mice per treatment group normalized to corresponding actin densitometry values. Vertical bars indicate standard errors of the means.
FIG. 7.
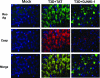
D-JNKI-1 treatment of T3D-infected mice decreases cleaved caspase-3. Adjacent tissue sections from mock- or T3D-infected mice treated with TAT control peptide or D-JNKI-1 were evaluated with dual-label fluorescence immunohistochemistry using antibody to σ3 reovirus protein (Reo Ag) (green) and cleavage specific (activated) caspase-3 (Casp) (red). Representative images are shown (original magnification, ×400) from the hippocampus of the T3D-infected groups. T3A data are not shown.
FIG. 8.
D-JNKI-1 treatment does not alter viral growth in the CNS. Two-day-old Swiss Webster mice were infected with T3A or T3D and treated with vehicle control or D-JNKI-1. Six hours following D-JNKI-1 or vehicle control treatment, mice were challenged with T3A and sacrificed on day 10 postinfection. Mice infected with T3D were treated with D-JNKI-1 or vehicle control 24 h postinfection and were sacrificed at day 8 postinfection. Each treatment group represents three replicates with six mice. Vertical bars indicate standard errors of the means.
Similar articles
- Fas-mediated apoptotic signaling in the mouse brain following reovirus infection.
Clarke P, Beckham JD, Leser JS, Hoyt CC, Tyler KL. Clarke P, et al. J Virol. 2009 Jun;83(12):6161-70. doi: 10.1128/JVI.02488-08. Epub 2009 Mar 25. J Virol. 2009. PMID: 19321603 Free PMC article. - Minocycline delays disease onset and mortality in reovirus encephalitis.
Richardson-Burns SM, Tyler KL. Richardson-Burns SM, et al. Exp Neurol. 2005 Apr;192(2):331-9. doi: 10.1016/j.expneurol.2004.11.015. Exp Neurol. 2005. PMID: 15755550 - Caspase-3 activation is required for reovirus-induced encephalitis in vivo.
Beckham JD, Tuttle KD, Tyler KL. Beckham JD, et al. J Neurovirol. 2010 Jul;16(4):306-17. doi: 10.3109/13550284.2010.499890. J Neurovirol. 2010. PMID: 20626234 Free PMC article. - Mechanisms of reovirus-induced cell death and tissue injury: role of apoptosis and virus-induced perturbation of host-cell signaling and transcription factor activation.
Clarke P, Debiasi RL, Goody R, Hoyt CC, Richardson-Burns S, Tyler KL. Clarke P, et al. Viral Immunol. 2005;18(1):89-115. doi: 10.1089/vim.2005.18.89. Viral Immunol. 2005. PMID: 15802955 Free PMC article. Review. - Inhibitors of c-Jun N-terminal kinases: JuNK no more?
Bogoyevitch MA, Arthur PG. Bogoyevitch MA, et al. Biochim Biophys Acta. 2008 Jan;1784(1):76-93. doi: 10.1016/j.bbapap.2007.09.013. Epub 2007 Oct 11. Biochim Biophys Acta. 2008. PMID: 17964301 Free PMC article. Review.
Cited by
- Bid regulates the pathogenesis of neurotropic reovirus.
Danthi P, Pruijssers AJ, Berger AK, Holm GH, Zinkel SS, Dermody TS. Danthi P, et al. PLoS Pathog. 2010 Jul 1;6(7):e1000980. doi: 10.1371/journal.ppat.1000980. PLoS Pathog. 2010. PMID: 20617182 Free PMC article. - The proapoptotic Bcl-2 protein Bax plays an important role in the pathogenesis of reovirus encephalitis.
Berens HM, Tyler KL. Berens HM, et al. J Virol. 2011 Apr;85(8):3858-71. doi: 10.1128/JVI.01958-10. Epub 2011 Feb 9. J Virol. 2011. PMID: 21307199 Free PMC article. - Fas-mediated apoptotic signaling in the mouse brain following reovirus infection.
Clarke P, Beckham JD, Leser JS, Hoyt CC, Tyler KL. Clarke P, et al. J Virol. 2009 Jun;83(12):6161-70. doi: 10.1128/JVI.02488-08. Epub 2009 Mar 25. J Virol. 2009. PMID: 19321603 Free PMC article. - Engagement of Neurotropic Viruses in Fast Axonal Transport: Mechanisms, Potential Role of Host Kinases and Implications for Neuronal Dysfunction.
Richards A, Berth SH, Brady S, Morfini G. Richards A, et al. Front Cell Neurosci. 2021 Jun 21;15:684762. doi: 10.3389/fncel.2021.684762. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34234649 Free PMC article. - JNK is activated but does not mediate hippocampal neuronal apoptosis in experimental neonatal pneumococcal meningitis.
Sury MD, Agarinis C, Widmer HR, Leib SL, Christen S. Sury MD, et al. Neurobiol Dis. 2008 Oct;32(1):142-50. doi: 10.1016/j.nbd.2008.07.006. Epub 2008 Jul 18. Neurobiol Dis. 2008. PMID: 18703144 Free PMC article.
References
- Barr, R. K., T. S. Kendrick, and M. A. Bogoyevitch. 2002. Identification of the critical features of a small peptide inhibitor of JNK activity. J. Biol. Chem. 277:10987-10997. - PubMed
- Bonny, C., A. Oberson, S. Negri, C. Sauser, and D. F. Schorderet. 2001. Cell-permeable peptide inhibitors of JNK: novel blockers of beta-cell death. Diabetes 50:77-82. - PubMed
- Borsello, T., P. G. Clarke, L. Hirt, A. Vercelli, M. Repici, D. F. Schorderet, J. Bogousslavsky, and C. Bonny. 2003. A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nat. Med. 9:1180-1186. - PubMed
- Borsello, T., K. Croquelois, J. P. Hornung, and P. G. Clarke. 2003. N-methyl-d-aspartate-triggered neuronal death in organotypic hippocampal cultures is endocytic, autophagic and mediated by the c-Jun N-terminal kinase pathway. Eur. J. Neurosci. 18:473-485. - PubMed
- Chang, L., Y. Jones, M. H. Ellisman, L. S. Goldstein, and M. Karin. 2003. JNK1 is required for maintenance of neuronal microtubules and controls phosphorylation of microtubule-associated proteins. Dev. Cell 4:521-533. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 5R01NS050138/NS/NINDS NIH HHS/United States
- T32 AI007537/AI/NIAID NIH HHS/United States
- R01 NS050138/NS/NINDS NIH HHS/United States
- R01 NS051403/NS/NINDS NIH HHS/United States
- 1R01NS051403/NS/NINDS NIH HHS/United States
- K08 AI076518/AI/NIAID NIH HHS/United States
- T32AI07537/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous