Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates - PubMed (original) (raw)
Comparative Study
. 2007 May 2;27(18):4894-901.
doi: 10.1523/JNEUROSCI.0237-07.2007.
Jeremy D Coplan, Sarah H Lisanby, Cecilia M Lipira, Mohamed Arif, Cristina Carpio, Gila Spitzer, Luca Santarelli, Bruce Scharf, Rene Hen, Gorazd Rosoklija, Harold A Sackeim, Andrew J Dwork
Affiliations
- PMID: 17475797
- PMCID: PMC6672102
- DOI: 10.1523/JNEUROSCI.0237-07.2007
Comparative Study
Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates
Tarique D Perera et al. J Neurosci. 2007.
Abstract
New neurons are generated in the adult hippocampus of many species including rodents, monkeys, and humans. Conditions associated with major depression, such as social stress, suppress hippocampal neurogenesis in rodents and primates. In contrast, all classes of antidepressants stimulate neuronal generation, and the behavioral effects of these medications are abolished when neurogenesis is blocked. These findings generated the hypothesis that induction of neurogenesis is a necessary component in the mechanism of action of antidepressant treatments. To date, the effects of antidepressants on newborn neurons have been reported only in rodents and tree shrews. This study examines whether neurogenesis is increased in nonhuman primates after antidepressant treatment. Adult monkeys received repeated electroconvulsive shock (ECS), which is the animal analog of electroconvulsive therapy (ECT), the most effective short-term antidepressant. Compared with control conditions, ECS robustly increased precursor cell proliferation in the subgranular zone (SGZ) of the dentate gyrus in the monkey hippocampus. A majority of these precursors differentiated into neurons or endothelial cells, while a few matured into glial cells. The ECS-mediated induction of cell proliferation and neurogenesis was accompanied by increased immunoreactivity for the neuroprotective gene product BCL2 (B cell chronic lymphocytic lymphoma 2) in the SGZ. The ECS interventions were not accompanied by increased hippocampal cell death or injury. This study demonstrates that ECS is capable of inducing neurogenesis in the nonhuman primate hippocampus and supports the possibility that antidepressant interventions produce similar alterations in the human brain.
Figures
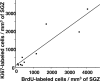
Figure 1.
Density of proliferating cells in the SGZ of the dentate gyrus of the hippocampus. A, Two raters, masked to the intervention, counted BrdU-labeled cells in monkeys randomized to intervention group (control, sham, ECS) and time the animal was killed (immediate-vs delayed-sacrifice). The intraclass correlation coefficient across all counts was 0.97. The density of BrdU-labeled cells in the SGZ was greater in the ECS group than the sham and control groups in the animals killed immediately after the treatment course (t = 6.3; df = 6; p = 0.001) and in animals killed 4 weeks after the completion of the interventions (t = 4.8; df = 2; p = 0.04). B, The density of Ki67-labeled cells in the SGZ was greater in the ECS group than the sham and control groups in the animals killed immediately after the treatment course (t = 3.4; df = 6; p = 0.01) and in animals killed 4 weeks after the completion of the interventions (t = 10.7; df = 2; p = 0.009).
Figure 2.
Correlation between BrdU- and Ki67-labeled cells. There was a positive correlation between densities of BrdU- and Ki67-labeled cells in the SGZ across all of the animals (ECS, sham, or control) killed immediately after the completion of interventions (r = 0.92; df = 8; p = 0.0002).
Figure 3.
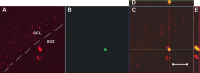
Images of BCL2-labeled cells in the SGZ. Expression of the BCL2 gene product (brown) in the dentate gyrus of animals killed immediately after the treatment course was significantly increased with ECS treatment. A, Sham-treated animal. B, ECS-treated animal. C, In both treated and untreated animals killed immediately and 4 weeks after intervention and BrdU injections, no colabeling of BrdU and BCL2 was identified in nickel-enhanced peroxidase double labeling. The picture shows cells in the SGZ of an ECS-treated monkey labeled for BCL2 in the cytoplasm (brown) and BrdU in the nucleus with nickel enhancement (black). Scale bars: A, 100 μm; C, 50 μm.
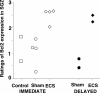
Figure 4.
Ratings of BCL2 expression in the SGZ. The expression of the BCL2 gene product in the SGZ was graded using a four-point semiquantitative scale by two independent raters masked to treatment conditions (intraclass correlation coefficient, 0.97). The BCL2 ratings were greater in the SGZ of ECS-treated monkeys compared with sham or controls in animals killed immediately after the treatment course (t = 4.5; df = 6; p = 0.004) and those killed 4 weeks after treatment (t = 8.0; df = 2; p = 0.02).
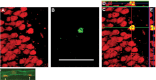
Figure 5.
Images of cells double labeled with BrdU/NeuN and BrdU/CD31. A–E, Confocal images of a newly formed neuron in the SGZ of the hippocampus of an ECS-treated monkey killed 4 weeks after treatment and BrdU injection. A, Red labels NeuN, a marker for mature neurons. B, Green labels BrdU, a marker of cell proliferation. C, Superimposed image of A and B. The double-labeled cells appear yellow. D, Confocal stack resliced in the x–z plane. E, Confocal stack resliced in the y–z plane. F, Fluorescent image of newly formed endothelial cells. Endothelial cytoplasm is labeled for CD31 (green) and two endothelial nuclei (indicated by arrows) in this capillary are labeled for BrdU (red). Scale bar: (in C) A–F, 10 μm.
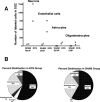
Figure 6.
Maturational fate of proliferating cells in the SGZ. A, B, The hippocampal sections of animals (2 ECS and 2 sham) killed 4 weeks after injection of BrdU were double-labeled for BrdU and maturational markers for mature neurons (NeuN), astroglia (GFAP), oligodendrocytes (CNP), or endothelial cells (CD31). Confocal analysis was conducted on a minimum of 25 BrdU-immunoreactive cells in at least three separate hippocampal sections to determine the percentage double labeled with each of the maturational markers. These percentages were then multiplied by the total number of BrdU cells per millimeters cubed of the SGZ that was previously determined using peroxidase methods. A, Graph showing the number of each mature cell-type for each animal. B, Pie charts showing the different percentages of mature cells in the ECS and sham groups.
Figure 7.
Confocal images of a cell double labeled with BrdU/calretinin in the SGZ. A–E, Confocal images of a newly generated calretinin-positive cell in the SGZ of an ECS-treated monkey killed immediately after the completion of treatment and BrdU injection. Calretinin is a Ca2+ binding protein typically seen in subpopulations of GABAergic interneurons, but this picture is of an immature granule cell, which transiently expresses calretinin during maturation. A, Red labels calretinin. B, Green labels BrdU. C, Superimposed image of A and B. D, Confocal stack resliced in the x–z plane. E, Confocal stack resliced in the y–z plane. Scale bar, 20 μm. GCL, Granule cell layer.
Similar articles
- Effects of single and repeated electroconvulsive stimulation on hippocampal cell proliferation and spontaneous behaviors in the rat.
Nakamura K, Ito M, Liu Y, Seki T, Suzuki T, Arai H. Nakamura K, et al. Brain Res. 2013 Jan 23;1491:88-97. doi: 10.1016/j.brainres.2012.10.052. Epub 2012 Nov 2. Brain Res. 2013. PMID: 23123207 - Antidepressant-like Effects of Electroconvulsive Seizures Require Adult Neurogenesis in a Neuroendocrine Model of Depression.
Schloesser RJ, Orvoen S, Jimenez DV, Hardy NF, Maynard KR, Sukumar M, Manji HK, Gardier AM, David DJ, Martinowich K. Schloesser RJ, et al. Brain Stimul. 2015 Sep-Oct;8(5):862-7. doi: 10.1016/j.brs.2015.05.011. Epub 2015 Jun 9. Brain Stimul. 2015. PMID: 26138027 Free PMC article. - Genetic fate mapping of type-1 stem cell-dependent increase in newborn hippocampal neurons after electroconvulsive seizures.
Weber T, Baier V, Lentz K, Herrmann E, Krumm B, Sartorius A, Kronenberg G, Bartsch D. Weber T, et al. Hippocampus. 2013 Dec;23(12):1321-30. doi: 10.1002/hipo.22171. Epub 2013 Sep 2. Hippocampus. 2013. PMID: 23893847 - Hippocampal neurogenesis, depressive disorders, and antidepressant therapy.
Paizanis E, Hamon M, Lanfumey L. Paizanis E, et al. Neural Plast. 2007;2007:73754. doi: 10.1155/2007/73754. Neural Plast. 2007. PMID: 17641737 Free PMC article. Review. - Brain ischemia, neurogenesis, and neurotrophic receptor expression in primates.
Tonchev AB. Tonchev AB. Arch Ital Biol. 2011 Jun;149(2):225-31. doi: 10.4449/aib.v149i2.1368. Arch Ital Biol. 2011. PMID: 21701994 Review.
Cited by
- Hippocampal neurogenesis in adult primates: a systematic review.
Elliott T, Liu KY, Hazan J, Wilson J, Vallipuram H, Jones K, Mahmood J, Gitlin-Leigh G, Howard R. Elliott T, et al. Mol Psychiatry. 2024 Nov 18. doi: 10.1038/s41380-024-02815-y. Online ahead of print. Mol Psychiatry. 2024. PMID: 39558003 - The electro-convulsive therapy story of Africa, a systematic review.
Abaatyo J, Kaggwa MM. Abaatyo J, et al. Discov Ment Health. 2024 Sep 9;4(1):31. doi: 10.1007/s44192-024-00085-2. Discov Ment Health. 2024. PMID: 39251508 Free PMC article. Review. - Neurogenesis-independent mechanisms of MRI-detectable hippocampal volume increase following electroconvulsive stimulation.
Abe Y, Yokoyama K, Kato T, Yagishita S, Tanaka KF, Takamiya A. Abe Y, et al. Neuropsychopharmacology. 2024 Jul;49(8):1236-1245. doi: 10.1038/s41386-023-01791-1. Epub 2024 Jan 9. Neuropsychopharmacology. 2024. PMID: 38195908 Free PMC article. - Neurogenesis in primates versus rodents and the value of non-human primate models.
Zhang R, Quan H, Wang Y, Luo F. Zhang R, et al. Natl Sci Rev. 2023 Sep 15;10(11):nwad248. doi: 10.1093/nsr/nwad248. eCollection 2023 Nov. Natl Sci Rev. 2023. PMID: 38025664 Free PMC article. Review.
References
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. - PubMed
- Bolwig TG, Hertz MM, Paulson OB, Spotoft H, Rafaelsen OJ. The permeability of the blood-brain barrier during electrically induced seizures in man. Eur J Clin Invest. 1977;7:87–93. - PubMed
- Bowdler JM, Green AR, Minchin MC, Nutt DJ. Regional GABA concentration and [3H]-diazepam binding in rat brain following repeated electroconvulsive shock. J Neural Transm. 1983;56:3–12. - PubMed
- Brandt MD, Jessberger S, Steiner B, Kronenberg G, Reuter K, Bick-Sander A, von der Behrens W, Kempermann G. Transient calretinin expression defines early postmitotic step of neuronal differentiation in adult hippocampal neurogenesis of mice. Mol Cell Neurosci. 2003;24:603–613. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 MH35636/MH/NIMH NIH HHS/United States
- R01MH59990/MH/NIMH NIH HHS/United States
- R01 MH035636/MH/NIMH NIH HHS/United States
- KO8 MH70954/MH/NIMH NIH HHS/United States
- R01 MH059990/MH/NIMH NIH HHS/United States
- K08 MH070954/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical