New transmembrane AMPA receptor regulatory protein isoform, gamma-7, differentially regulates AMPA receptors - PubMed (original) (raw)
Comparative Study
New transmembrane AMPA receptor regulatory protein isoform, gamma-7, differentially regulates AMPA receptors
Akihiko S Kato et al. J Neurosci. 2007.
Abstract
AMPA-type glutamate receptors (GluRs) mediate most excitatory signaling in the brain and are composed of GluR principal subunits and transmembrane AMPA receptor regulatory protein (TARP) auxiliary subunits. Previous studies identified four mammalian TARPs, gamma-2 (or stargazin), gamma-3, gamma-4, and gamma-8, that control AMPA receptor trafficking, gating, and pharmacology. Here, we explore roles for the homologous gamma-5 and gamma-7 proteins, which were previously suggested not to serve as TARPs. Western blotting reveals high levels of gamma-5 and gamma-7 in the cerebellum, where gamma-7 is enriched in Purkinje neurons in the molecular layer and glomerular synapses in the granule cell layer. Immunoprecipitation proteomics shows that cerebellar gamma-7 avidly and selectively binds to AMPA receptor GluR subunits and also binds to the AMPA receptor clustering protein, postsynaptic density-95 (PSD-95). Furthermore, gamma-7 occurs together with PSD-95 and AMPA receptor subunits in purified postsynaptic densities. In heterologous cells, gamma-7 but not gamma-5 greatly enhances AMPA receptor glutamate-evoked currents and modulates channel gating. In granule cells from stargazer mice, transfection of gamma-7 but not gamma-5 increases AMPA receptor-mediated currents. Compared with stargazin, gamma-7 differentially modulates AMPA receptor glutamate affinity and kainate efficacy. These studies define gamma-7 as a new member of the TARP family that can differentially influence AMPA receptors in cerebellar neurons.
Figures
Figure 1.
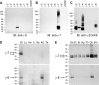
γ-5 and γ-7 are enriched in cerebellum. A–C, Specificity of anti-γ-5 and γ-7 antibodies. Extracts from HEK 293T cells transiently transfected with cDNAs encoding γ subunits were separated by SDS-PAGE and immunoblotted with antibodies to γ-5, γ-7, and γ-2. A, B, The γ-5 and γ-7 antibodies label only their appropriate protein product. C, The anti-γ-2 antibody cross-reacts with γ-3, γ-4, and γ-8. IB, Immunoblotting. D, Tissue distribution of γ-5 and γ-7. Postnuclear membrane fractions (5 μg) were blotted with antibodies as indicated. γ-5 was detected in the brain and kidney. γ-7 was detected only in the brain. Br, Brain; Lu, lung; He, heart; Li, liver; Sp, spleen; Kd, kidney; Te, testis. E, Expression profile of γ-5 and γ-7 in the rat brain. γ-5 and γ-7 proteins were enriched in the cerebellum and also occur in the olfactory bulb. In addition, γ-7 occurs at low levels in all other brain regions surveyed. Postnuclear membrane fractions (9 μg) were immunoblotted. Ob, Olfactory bulb; Ct, cerebral cortex; St, striatum; Hp, hippocampus; Th, Thalamus; Cb, cerebellum; Sm, brainstem.
Figure 2.
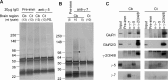
AMPA receptor subunits are the major binding partners for γ-7. A, B, Solubilized cerebellar or cerebrocortical membranes were immunoprecipitated with antibodies to γ-5 (A) or γ-7 (B), and adherent proteins were analyzed by silver staining. We detected a specific band at 100 kDa (arrowhead) in the γ-7 immunoprecipitate from the cerebellum. No specific bands were identified in γ-7 immunoprecipitates from the cortex or in γ-5-immunoprecipitates from either brain region. Mass spectroscopy revealed that the γ-7-associated 100 kDa band contained GluR1, 2, 3, and 4. The 30–40 kDa region (bar) in γ-7-immunoprecipitates from the cerebellum containing γ-7. C, Western blotting analysis of the immunoprecipitates. AMPA receptor subunits, GluR1 and GluR2/3, were readily detected in the γ-7 immunoprecipitates from the cerebellum, but were faintly detected from the cortex, which is consistent with cerebellar enrichment of γ-7. GluR1 was weakly detected in γ-5 immunoprecipitate from the cerebellum. Minimal coimmunoprecipitation was noted among the γ subunits. Input represents 0.2% of the total, which explains lack of input signal in GluR1 and γ-2/3/4/8, -5, and -7. Cb, Cerebellum; Ct, cortex; NL, no brain lysate; pre-imm, preimmune serum.
Figure 3.
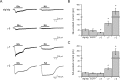
γ-7 functionally regulates AMPA receptors. HEK 293T cells were cotransfected with GluR1 and γ-2 or γ-7, and agonist-evoked currents were recorded. A, Typical traces of glutamate- (300 μ
m
) or kainate- (500 μ
m
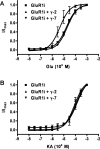
) evoked responses. Calibration: 200 pA, 2 s. B, C, γ-7 increased the glutamate-evoked response of GluR1. Steady-state currents evoked by glutamate in the following GluR1i (B) or GluR1o (C): GluR1i, 44.3 ± 10.9 pA (n = 13); GluR1i plus γ-2, 399 ± 78 pA (n = 10); GluR1i plus γ-5, 85.7 ± 36.3 (n = 7); GluR1i plus γ-6, 27.3 ± 7.9 (n = 8); GluR1i plus γ-7, 423 ± 88 pA (n = 23); GluR1o, 1.27 ± 0.36 pA (n = 6); GluRo plus γ-2, 25.6 ± 11.2 pA (n = 7); GluR1o plus γ-7, 11.6 ± 4.6 pA (n = 7). D, E, γ-7 increased kainate-evoked responses from GluR1. Steady-state currents evoked by kainate in the following GluR1i (D) or GluR1o (E): GluR1i, 33.3 ± 9.4 pA (n = 11); GluR1i plus γ-2, 8252 ± 2578 pA (n = 8); GluR1i plus γ-5, 120.7 ± 50.3 (n = 7); GluR1i plus γ-6, 21.2 ± 5.6 (n = 8); GluRi plus γ-7, 1969 ± 498 pA (n = 9); GluR1o, 2.37 ± 1.5 pA (n = 6); GluRo plus γ-2, 3147 ± 1014 pA (n = 7); GluR1o plus γ-7, 124.6 ± 52.5 pA (n = 7). F–I, γ-7 and γ-2 differentially regulate GluR1 function. The cells with GluR1i (F) or GluR1o (G) were treated with glutamate and then with kainate. Sequential change in the amplitude of steady-state currents was plotted. Both γ-7 and γ-2 gave larger response to kainate than to glutamate. The ratio of kainate- to glutamate-evoked currents in GluR1i (H) or GluR1o (I) shows that γ-2 makes GluR1 more sensitive to kainate than does γ-7. Error bars indicate SEM.
Figure 4.
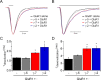
γ-2, but not γ-7, lowers the EC50 for glutamate at GluR1i. Dose-response curve of glutamate- (A) or kainate- (KA; B) evoked currents in transfected 293T cells. The steady-state currents evoked by varying concentrations of glutamate or kainate were measured and normalized to that evoked by 1 m
m
glutamate or kainate. Error bars indicate SEM.
Figure 5.
γ-7, but not γ-5, slows deactivation and desensitization of GluR1 responses. HEK293T cells were transfected with GluR1i either alone or with γ-5, γ-7, or γ-2, and glutamate-evoked currents from outside-out patches were recorded. A, Superimposed typical responses to 1 ms (A) or 100 ms (B) applications of 1 m
m
glutamate. Responses were normalized to their peak amplitudes to compare their time courses. C, γ-7 (n = 8) and γ-2 (n = 5) slowed deactivation of GluR1i (n = 5) (p < 0.01), whereas γ-5 (n = 3) did not. D, γ-7 (n = 5) and γ-2 slowed deactivation of GluR1i (n = 4) (p < 0.01), whereas γ-5 (n = 4) did not. Error bars indicate SEM. Asterisks indicate statistical significance with respect to GluR1i alone (unpaired Wilcoxon test with corrected α).
Figure 6.
γ-7, but not γ-5, rescued the surface expression of AMPA receptors in stargazer cerebellar granule cells. Cerebellar granule neurons from stg/stg mice were transfected with GFP, γ-5, γ-7, or γ-2, and agonist-evoked currents were recorded. A, Typical traces of responses to 1 m
m
glutamate plus 100 μ
m
cyclothiazide or 1 m
m
kainate (KA). B,C, γ-7 (n = 15) and γ-2 (n = 11) potentiated AMPA receptor responses to both glutamate (p < 0.005) and kainate (p < 0.0001) to different extents relative to untransfected (n = 15) or GFP (n = 12), whereas γ-5 (n = 12) reduced responses to glutamate (p < 0.002). Error bars indicate SEM. Asterisks indicate statistical significance with respect to untransfected (unpaired Wilcoxon test with corrected α).
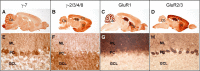
Figure 7.
Differential localization of γ-7 and γ-2/3/4/8 in the brain. Sagittal brain sections were stained with antibodies to γ-7 (A, E), γ-2/3/4/8 (B, F), GluR1 (C, G), or GluR2 (D, H). γ-7 is enriched in the cerebellum (A) and γ-2/3/4/8 stains numerous brain regions (B). GluR1 and GluR2 both occur discretely in neuronal populations throughout in the brain (C, D). Cb, cerebellum; Ct, cerebral cortex; Hp, hippocampus; Sp, septum; St, striatum. E, High-power magnification shows γ-7 immunoreactivity in the somatodendritic regions of Purkinje cells and in glomeruli of the granule cell layer. F, Antibody to γ-2/3/4/8 stained all layers in cerebellum. G, GluR1 gave strong staining in molecular layer. H, GluR2 showed strong signal in Purkinje cells and lesser staining in molecular and granule cell layers. ML, Molecular layer; PC, Purkinje cell; GCL, granule cell layer.
Figure 8.
γ-7 is enriched in cerebellar postsynaptic densities and binds to PSD-95. A, Subcellular fractions from rat cerebellum were assayed by Western blotting. γ-7 and γ-2/3/4/8 cofractionate with GluR1 and GluR2/3 and are enriched in the PSD, whereas γ-5 does not. PSD-95 and synaptophysin served as markers of PSD and synaptic vesicles, respectively. H, Homogenate; P1′, crude nuclear fraction; P2′, crude synaptosomal fraction; S4, cytosolic fraction; LP1, lysate heavy membrane pellet; LP2, cytosolic synaptosomal pellet; LS2, cytosolic synaptosomal supernatant; Syn, synaptosome; PSD-I and PSD-II, postsynaptic densities extracted with 0.5% Triton X-100 once and twice, respectively. B, Differential solubility of γ-7 and γ-5 in cerebellum. Rat cerebellar membranes were incubated with Triton X-100, and the supernatant (S) and pellet (P) after centrifugation were probed for γ-5 and γ-7. γ-7 is more insoluble than γ-5, supporting the differential subcellular localization of γ-7 and γ-5. C, Brain lysates were immunoprecipitated with antibodies (α-) or preimmune (pre-imm) as indicated, and then immunoblotted with anti-PSD-95 antibody. PSD-95 was detected in the coimmunoprecipitate with γ-2/3/4/8 and γ-7. Cb, Cerebellum; Ct, cortex. IP, immunoprecipitation; IB, immunoblotting. D, HEK 293T cells expressing γ-subunits and PSD-95 were immunoprecipitated with antibody to PSD-95 and were blotted with the indicated antibodies. γ-2 and γ-7 but not γ-1 coimmunoprecipitated with PSD-95.
Similar articles
- TARPs gamma-2 and gamma-7 are essential for AMPA receptor expression in the cerebellum.
Yamazaki M, Fukaya M, Hashimoto K, Yamasaki M, Tsujita M, Itakura M, Abe M, Natsume R, Takahashi M, Kano M, Sakimura K, Watanabe M. Yamazaki M, et al. Eur J Neurosci. 2010 Jun;31(12):2204-20. doi: 10.1111/j.1460-9568.2010.07254.x. Epub 2010 Jun 7. Eur J Neurosci. 2010. PMID: 20529126 - AMPA receptor subunit-specific regulation by a distinct family of type II TARPs.
Kato AS, Siuda ER, Nisenbaum ES, Bredt DS. Kato AS, et al. Neuron. 2008 Sep 25;59(6):986-96. doi: 10.1016/j.neuron.2008.07.034. Neuron. 2008. PMID: 18817736 - AMPA receptor modulation by cornichon-2 dictated by transmembrane AMPA receptor regulatory protein isoform.
Gill MB, Kato AS, Wang H, Bredt DS. Gill MB, et al. Eur J Neurosci. 2012 Jan;35(2):182-94. doi: 10.1111/j.1460-9568.2011.07948.x. Epub 2011 Dec 30. Eur J Neurosci. 2012. PMID: 22211840 - Transmembrane AMPA receptor regulatory proteins and AMPA receptor function in the cerebellum.
Coombs ID, Cull-Candy SG. Coombs ID, et al. Neuroscience. 2009 Sep 1;162(3):656-65. doi: 10.1016/j.neuroscience.2009.01.004. Epub 2009 Jan 13. Neuroscience. 2009. PMID: 19185052 Free PMC article. Review. - TARPs differentially decorate AMPA receptors to specify neuropharmacology.
Kato AS, Gill MB, Yu H, Nisenbaum ES, Bredt DS. Kato AS, et al. Trends Neurosci. 2010 May;33(5):241-8. doi: 10.1016/j.tins.2010.02.004. Epub 2010 Mar 8. Trends Neurosci. 2010. PMID: 20219255 Review.
Cited by
- Rapid sequential clustering of NMDARs, CaMKII, and AMPARs upon activation of NMDARs at developing synapses.
Chen Y, Liu S, Jacobi AA, Jeng G, Ulrich JD, Stein IS, Patriarchi T, Hell JW. Chen Y, et al. Front Synaptic Neurosci. 2024 Apr 10;16:1291262. doi: 10.3389/fnsyn.2024.1291262. eCollection 2024. Front Synaptic Neurosci. 2024. PMID: 38660466 Free PMC article. - GSG1L-containing AMPA receptor complexes are defined by their spatiotemporal expression, native interactome and allosteric sites.
Perozzo AM, Schwenk J, Kamalova A, Nakagawa T, Fakler B, Bowie D. Perozzo AM, et al. Nat Commun. 2023 Oct 26;14(1):6799. doi: 10.1038/s41467-023-42517-7. Nat Commun. 2023. PMID: 37884493 Free PMC article. - Anatomical Diversity of the Adult Corticospinal Tract Revealed by Single-Cell Transcriptional Profiling.
Golan N, Ehrlich D, Bonanno J, O'Brien RF, Murillo M, Kauer SD, Ravindra N, Van Dijk D, Cafferty WB. Golan N, et al. J Neurosci. 2023 Nov 22;43(47):7929-7945. doi: 10.1523/JNEUROSCI.0811-22.2023. J Neurosci. 2023. PMID: 37748862 Free PMC article. - Modulation of GluA2-γ5 synaptic complex desensitization, polyamine block and antiepileptic perampanel inhibition by auxiliary subunit cornichon-2.
Gangwar SP, Yen LY, Yelshanskaya MV, Korman A, Jones DR, Sobolevsky AI. Gangwar SP, et al. Nat Struct Mol Biol. 2023 Oct;30(10):1481-1494. doi: 10.1038/s41594-023-01080-x. Epub 2023 Aug 31. Nat Struct Mol Biol. 2023. PMID: 37653241 Free PMC article. - Involvement of CaV 2.2 channels and α2 δ-1 in homeostatic synaptic plasticity in cultured hippocampal neurons.
Pilch KS, Ramgoolam KH, Dolphin AC. Pilch KS, et al. J Physiol. 2022 Dec;600(24):5333-5351. doi: 10.1113/JP283600. Epub 2022 Dec 3. J Physiol. 2022. PMID: 36377048 Free PMC article.
References
- Allen Institute for Brain Science. Allen Brain Atlas. 2007. Retrieved April 18, 2007, from: http://www.brainmap.org.
- Bedoukian MA, Weeks AM, Partin KM. Different domains of the AMPA receptor direct stargazin-mediated trafficking and stargazin-mediated modulation of kinetics. J Biol Chem. 2006;281:23908–23921. - PubMed
- Burgess DL, Gefrides LA, Foreman PJ, Noebels JL. A cluster of three novel Ca2+ channel gamma subunit genes on chromosome 19q13.4: evolution and expression profile of the gamma subunit gene family. Genomics. 2001;71:339–350. - PubMed
- Chen L, El-Husseini A, Tomita S, Bredt DS, Nicoll RA. Stargazin differentially controls the trafficking of alpha-amino-3-hydroxyl-5-methyl-4-isoxazolepropionate and kainate receptors. Mol Pharmacol. 2003;64:703–706. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases