Nutrient signalling in the regulation of human muscle protein synthesis - PubMed (original) (raw)
Nutrient signalling in the regulation of human muscle protein synthesis
Satoshi Fujita et al. J Physiol. 2007.
Abstract
The mammalian target of rapamycin (mTOR) and AMP-activated protein kinase (AMPK) are important nutrient- and energy-sensing and signalling proteins in skeletal muscle. AMPK activation decreases muscle protein synthesis by inhibiting mTOR signalling to regulatory proteins associated with translation initiation and elongation. On the other hand, essential amino acids (leucine in particular) and insulin stimulate mTOR signalling and protein synthesis. We hypothesized that anabolic nutrients would be sensed by both AMPK and mTOR, resulting in an acute and potent stimulation of human skeletal muscle protein synthesis via enhanced translation initiation and elongation. We measured muscle protein synthesis and mTOR-associated upstream and downstream signalling proteins in young male subjects (n=14) using stable isotopic and immunoblotting techniques. Following a first muscle biopsy, subjects in the 'Nutrition' group ingested a leucine-enriched essential amino acid-carbohydrate mixture (EAC). Subjects in the Control group did not consume nutrients. A second biopsy was obtained 1 h later. Ingestion of EAC significantly increased muscle protein synthesis, modestly reduced AMPK phosphorylation, and increased Akt/PKB (protein kinase B) and mTOR phosphorylation (P<0.05). mTOR signalling to its downstream effectors (S6 kinase 1 (S6K1) and 4E-binding protein 1 (4E-BP1) phosphorylation status) was also increased (P<0.05). In addition, eukaryotic elongation factor 2 (eEF2) phosphorylation was significantly reduced (P<0.05). Protein synthesis and cell signalling (phosphorylation status) was unchanged in the control group (P>0.05). We conclude that anabolic nutrients alter the phosphorylation status of both AMPK- and mTOR-associated signalling proteins in human muscle, in association with an increase in protein synthesis not only via enhanced translation initiation but also through signalling promoting translation elongation.
Figures
Figure 1. Substrate and hormone kinetics following nutrient intake
Control data are from fasting subjects. Nutrition data are from subjects following an ingestion of essential amino acids and carbohydrate. Following nutrient ingestion: A, arterial glucose and insulin concentrations; B, glucose uptake across the leg; C, arterial phenylalanine concentration; D, muscle free phenylalanine and leucine concentrations. *P < 0.05 versus Control.
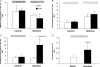
Figure 2. Phosphorylation status of upstream regulators of mTOR
Data in A_–_C are presented as Pre and Post. Pre data are from the first muscle biopsy in both the Control and Nutrition groups and indicate fasting phosphorylation status. Post data are from the second muscle biopsy obtained 1 h later. In the Control group no nutrients were ingested and the second biopsy was able to assess if there was any effect of the biopsy procedure on phosphorylation status. The Post data in the Nutrition group reflect the change in phosphorylation status 1 h after ingestion of essential amino acids and carbohydrate. A, AMPKα phosphorylation at Thr 172; B, TSC2 phosphorylation at Thr 1462; C, Akt/PKB phosphorylation at Ser 473; D, IRS-1 Ser 312 phosphorylation in the Nutrition group at baseline (basal) and 1 h after nutrient ingestion (Nutrition). *P < 0.05 versus Pre.
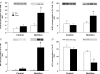
Figure 3. Phosphorylation status of downstream components of the mTOR signalling pathway
Data are presented as Pre and Post. Pre data are from the first muscle biopsy in both the Control and Nutrition groups and indicate fasting phosphorylation status. Post data are from the second muscle biopsy obtained 1 h later. In the Control group no nutrients were ingested and the second biopsy was able to assess if there was any effect of the biopsy procedure on phosphorylation status. The Post data in the Nutrition group reflect the change in phosphorylation status 1 h after ingestion of essential amino acids and carbohydrate. A, mTOR phosphorylation at Ser 2448; B, 4E-BP1 phosphorylation at Thr 37/46; C, S6K1 phosphorylation at Thr 389; D, eEF2 phosphorylation at Thr 56. *P < 0.05 versus Pre.
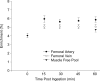
Figure 4. Phenylalanine enrichmnts (tracer/tracee) in the femoral artery and vein during the hour following nutrient ingestion
Blood enrichments were stable throughout the experiment and were not different between time points (P > 0.05). Enrichments were also stable in the Control group (data not shown) and were not different from the Nutrition group (P > 0.05). Intracellular phenylalanine enrichment in the muscle free pool is shown for the biopsy collected immediately before and 1 h following nutrient ingestion. Muscle free pool enrichment was not different between the two time points (P > 0.05).
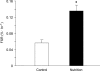
Figure 5. Muscle protein synthesis (FSR)
The basal FSR in the Control group is the synthesis rate in the absence of nutrients. Nutrition data reflect the large increase in muscle protein synthesis following an ingestion of essential amino acids and carbohydrate. *P < 0.05 versus Control.
Similar articles
- Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle.
Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Dreyer HC, et al. J Physiol. 2006 Oct 15;576(Pt 2):613-24. doi: 10.1113/jphysiol.2006.113175. Epub 2006 Jul 27. J Physiol. 2006. PMID: 16873412 Free PMC article. - Developmental regulation of the activation of signaling components leading to translation initiation in skeletal muscle of neonatal pigs.
Suryawan A, Escobar J, Frank JW, Nguyen HV, Davis TA. Suryawan A, et al. Am J Physiol Endocrinol Metab. 2006 Oct;291(4):E849-59. doi: 10.1152/ajpendo.00069.2006. Epub 2006 Jun 6. Am J Physiol Endocrinol Metab. 2006. PMID: 16757550 - Leucine stimulates protein synthesis in skeletal muscle of neonatal pigs by enhancing mTORC1 activation.
Suryawan A, Jeyapalan AS, Orellana RA, Wilson FA, Nguyen HV, Davis TA. Suryawan A, et al. Am J Physiol Endocrinol Metab. 2008 Oct;295(4):E868-75. doi: 10.1152/ajpendo.90314.2008. Epub 2008 Aug 5. Am J Physiol Endocrinol Metab. 2008. PMID: 18682538 Free PMC article. - Leucine-enriched nutrients and the regulation of mammalian target of rapamycin signalling and human skeletal muscle protein synthesis.
Drummond MJ, Rasmussen BB. Drummond MJ, et al. Curr Opin Clin Nutr Metab Care. 2008 May;11(3):222-6. doi: 10.1097/MCO.0b013e3282fa17fb. Curr Opin Clin Nutr Metab Care. 2008. PMID: 18403916 Free PMC article. Review. - Role of mTOR signalling in the control of translation initiation and elongation by nutrients.
Proud CG. Proud CG. Curr Top Microbiol Immunol. 2004;279:215-44. doi: 10.1007/978-3-642-18930-2_13. Curr Top Microbiol Immunol. 2004. PMID: 14560960 Review.
Cited by
- Is the optimal level of protein intake for older adults greater than the recommended dietary allowance?
Volpi E, Campbell WW, Dwyer JT, Johnson MA, Jensen GL, Morley JE, Wolfe RR. Volpi E, et al. J Gerontol A Biol Sci Med Sci. 2013 Jun;68(6):677-81. doi: 10.1093/gerona/gls229. Epub 2012 Nov 26. J Gerontol A Biol Sci Med Sci. 2013. PMID: 23183903 Free PMC article. Review. - Whey protein intake after resistance exercise activates mTOR signaling in a dose-dependent manner in human skeletal muscle.
Kakigi R, Yoshihara T, Ozaki H, Ogura Y, Ichinoseki-Sekine N, Kobayashi H, Naito H. Kakigi R, et al. Eur J Appl Physiol. 2014 Apr;114(4):735-42. doi: 10.1007/s00421-013-2812-7. Epub 2014 Jan 3. Eur J Appl Physiol. 2014. PMID: 24384983 - Metabolic Remodeling in Skeletal Muscle Atrophy as a Therapeutic Target.
Renzini A, Riera CS, Minic I, D'Ercole C, Lozanoska-Ochser B, Cedola A, Gigli G, Moresi V, Madaro L. Renzini A, et al. Metabolites. 2021 Aug 5;11(8):517. doi: 10.3390/metabo11080517. Metabolites. 2021. PMID: 34436458 Free PMC article. Review. - Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle.
Greenhaff PL, Karagounis LG, Peirce N, Simpson EJ, Hazell M, Layfield R, Wackerhage H, Smith K, Atherton P, Selby A, Rennie MJ. Greenhaff PL, et al. Am J Physiol Endocrinol Metab. 2008 Sep;295(3):E595-604. doi: 10.1152/ajpendo.90411.2008. Epub 2008 Jun 24. Am J Physiol Endocrinol Metab. 2008. PMID: 18577697 Free PMC article. - Daily Protein and Energy Intake Are Not Associated with Muscle Mass and Physical Function in Healthy Older Individuals-A Cross-Sectional Study.
Højfeldt G, Nishimura Y, Mertz K, Schacht SR, Lindberg J, Jensen M, Hjulmand M, Lind MV, Jensen T, Jespersen AP, Reitelseder S, Tetens I, Holm L. Højfeldt G, et al. Nutrients. 2020 Sep 12;12(9):2794. doi: 10.3390/nu12092794. Nutrients. 2020. PMID: 32932629 Free PMC article.
References
- Anthony JC, Lang CH, Crozier SJ, Anthony TG, MacLean DA, Kimball SR, Jefferson LS. Contribution of insulin to the translational control of protein synthesis in skeletal muscle by leucine. Am J Physiol Endocrinol Metab. 2002;282:E1092–E1101. - PubMed
- Avruch J, Hara K, Lin Y, Liu M, Long X, Ortiz-Vega S, Yonezawa K. Insulin and amino-acid regulation of mTOR signaling and kinase activity through the Rheb GTPase. Oncogene. 2006;25:6361–6372. - PubMed
- Avruch J, Lin Y, Long X, Murthy S, Ortiz-Vega S. Recent advances in the regulation of the TOR pathway by insulin and nutrients. Curr Opin Clin Nutr Metab Care. 2005;8:67–72. - PubMed
- Baar K, Esser K. Phosphorylation of p70 (S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol Cell Physiol. 1999;276:C120–C127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- S10 RR16650/RR/NCRR NIH HHS/United States
- M01 RR00073/RR/NCRR NIH HHS/United States
- R01 AR049877/AR/NIAMS NIH HHS/United States
- M01 RR000073/RR/NCRR NIH HHS/United States
- P30 AG024832/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous