Roles of cyclic diguanylate in the regulation of bacterial pathogenesis - PubMed (original) (raw)
Review
Roles of cyclic diguanylate in the regulation of bacterial pathogenesis
Rita Tamayo et al. Annu Rev Microbiol. 2007.
Abstract
Cyclic diguanylate (c-di-GMP) is a bacterial second messenger of growing recognition involved in the regulation of a number of complex physiological processes. This review describes the biosynthesis and hydrolysis of c-di-GMP and several mechanisms of regulation of c-di-GMP metabolism. The contribution of c-di-GMP to regulating biofilm formation and motility, processes that affect pathogenesis of many bacteria, is described, as is c-di-GMP regulation of virulence gene expression. Finally, ways in which c-di-GMP may mediate these regulatory effects are proposed.
Figures
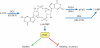
Figure 1
c-di-GMP regulatory pathway. c-di-GMP is synthesized from two GTPs by GGDEF domain DGCs. c-di-GMP is hydrolyzed by EAL domain PDEAs into the linear pGpG before being hydrolyzed by other PDEs into two GMPs. HD-GYP domain PDEs hydrolyze c-di-GMP completely into two GMPs. Numerous studies have shown that c-di-GMP activates EPS production and biofilm formation but inhibits motility and virulence. These processes are regulated by c-di-GMP through a variety of pathways, as discussed in the text. PilZ domain proteins are one class of c-di-GMP sensor, although others likely exist. The mechanism(s) by which PilZ domain proteins transduce changes in c-di-GMP into downstream physiological effects is unknown.
Figure 2
(a) Model of the role of c-di-GMP in the transition of V. cholerae from persistence in aquatic reservoirs to survival in the human host. c-di-GMP is predicted to be high in V. cholerae existing in biofilms attached to biotic and abiotic surfaces in the pond environment. Upon entry into the human host, induction of PDE and/or repression of DGC activities can lower c-di-GMP and allow dispersion from the biofilm and maximal expression of virulence genes. Conversely, c-di-GMP must be elevated once again through activation of DGC and/or repression of PDE to resume the biofilm lifestyle. There is evidence that c-di-GMP may begin to be elevated during the later stage of V. cholerae infection, potentially giving the bacteria an advantage once they have exited the host. (b) The VieSA signal transduction system provides one mechanism by which V. cholerae can lower c-di-GMP concentration in the small intestine. An unknown extracellular signal present in the small intestine activates the VieS sensor, leading to autophosphorylation of VieS and phosphotransfer to the dual-function protein VieA. The phosphorylated, activated VieA autoactivates vieSA transcription, resulting in higher VieA PDEA in the cell, reduced c-di-GMP, and full expression of virulence factors.
Similar articles
- BolA Is Required for the Accurate Regulation of c-di-GMP, a Central Player in Biofilm Formation.
Moreira RN, Dressaire C, Barahona S, Galego L, Kaever V, Jenal U, Arraiano CM. Moreira RN, et al. mBio. 2017 Sep 19;8(5):e00443-17. doi: 10.1128/mBio.00443-17. mBio. 2017. PMID: 28928205 Free PMC article. - Cyclic Diguanylate Regulates Virulence Factor Genes via Multiple Riboswitches in Clostridium difficile.
McKee RW, Harvest CK, Tamayo R. McKee RW, et al. mSphere. 2018 Oct 24;3(5):e00423-18. doi: 10.1128/mSphere.00423-18. mSphere. 2018. PMID: 30355665 Free PMC article. - Systematic Analysis of c-di-GMP Signaling Mechanisms and Biological Functions in Dickeya zeae EC1.
Chen Y, Zhou J, Lv M, Liang Z, Parsek MR, Zhang LH. Chen Y, et al. mBio. 2020 Dec 1;11(6):e02993-20. doi: 10.1128/mBio.02993-20. mBio. 2020. PMID: 33262261 Free PMC article. - Cyclic di-GMP: the first 25 years of a universal bacterial second messenger.
Römling U, Galperin MY, Gomelsky M. Römling U, et al. Microbiol Mol Biol Rev. 2013 Mar;77(1):1-52. doi: 10.1128/MMBR.00043-12. Microbiol Mol Biol Rev. 2013. PMID: 23471616 Free PMC article. Review. - Multiple Roles of c-di-GMP Signaling in Bacterial Pathogenesis.
Valentini M, Filloux A. Valentini M, et al. Annu Rev Microbiol. 2019 Sep 8;73:387-406. doi: 10.1146/annurev-micro-020518-115555. Annu Rev Microbiol. 2019. PMID: 31500536 Review.
Cited by
- Cyclic Di-GMP phosphodiesterases RmdA and RmdB are involved in regulating colony morphology and development in Streptomyces coelicolor.
Hull TD, Ryu MH, Sullivan MJ, Johnson RC, Klena NT, Geiger RM, Gomelsky M, Bennett JA. Hull TD, et al. J Bacteriol. 2012 Sep;194(17):4642-51. doi: 10.1128/JB.00157-12. Epub 2012 Jun 29. J Bacteriol. 2012. PMID: 22753061 Free PMC article. - Engineered allosteric ribozymes that sense the bacterial second messenger cyclic diguanosyl 5'-monophosphate.
Gu H, Furukawa K, Breaker RR. Gu H, et al. Anal Chem. 2012 Jun 5;84(11):4935-41. doi: 10.1021/ac300415k. Epub 2012 May 21. Anal Chem. 2012. PMID: 22519888 Free PMC article. - A study of Chitosan and c-di-GMP as mucosal adjuvants for intranasal influenza H5N1 vaccine.
Svindland SC, Pedersen GK, Pathirana RD, Bredholt G, Nøstbakken JK, Jul-Larsen Å, Guzmán CA, Montomoli E, Lapini G, Piccirella S, Jabbal-Gill I, Hinchcliffe M, Cox RJ. Svindland SC, et al. Influenza Other Respir Viruses. 2013 Nov;7(6):1181-93. doi: 10.1111/irv.12056. Epub 2012 Nov 21. Influenza Other Respir Viruses. 2013. PMID: 23170900 Free PMC article. - Rapid microevolution of biofilm cells in response to antibiotics.
Penesyan A, Nagy SS, Kjelleberg S, Gillings MR, Paulsen IT. Penesyan A, et al. NPJ Biofilms Microbiomes. 2019 Nov 6;5(1):34. doi: 10.1038/s41522-019-0108-3. eCollection 2019. NPJ Biofilms Microbiomes. 2019. PMID: 31728201 Free PMC article. - Metagenomics Analysis to Investigate the Microbial Communities and Their Functional Profile During Cyanobacterial Blooms in Lake Varese.
Sanseverino I, Pretto P, António DC, Lahm A, Facca C, Loos R, Skejo H, Beghi A, Pandolfi F, Genoni P, Lettieri T. Sanseverino I, et al. Microb Ecol. 2022 May;83(4):850-868. doi: 10.1007/s00248-021-01914-5. Epub 2021 Nov 12. Microb Ecol. 2022. PMID: 34766210 Free PMC article.
References
- Aldridge P, Paul R, Goymer P, Rainey P, Jenal U. Role of the GGDEF regulator PleD in polar development of Caulobacter crescentus. Mol. Microbiol. 2003;47:1695–708. - PubMed
- Amikam D, Galperin MY. PilZ domain is part of the bacterial c-di-GMP binding protein. Bioinformatics. 2006;22:3–6. [Uses bioinformatics to correctly predict a c-di-GMP binding protein domain called PilZ.] - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 AI055058/AI/NIAID NIH HHS/United States
- R01 AI055058/AI/NIAID NIH HHS/United States
- R01 AI055058-02/AI/NIAID NIH HHS/United States
- R01 AI045746-06A1/AI/NIAID NIH HHS/United States
- R01 AI045746/AI/NIAID NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources