Synergy between PPARgamma ligands and platinum-based drugs in cancer - PubMed (original) (raw)
Synergy between PPARgamma ligands and platinum-based drugs in cancer
Geoffrey D Girnun et al. Cancer Cell. 2007 May.
Abstract
PPARgamma is a member of the nuclear receptor family for which agonist ligands have antigrowth effects. However, clinical studies using PPARgamma ligands as a monotherapy failed to show a beneficial effect. Here we have studied the effects of PPARgamma activation with chemotherapeutic agents in current use for specific cancers. We observed a striking synergy between rosiglitazone and platinum-based drugs in several different cancers both in vitro and using transplantable and chemically induced "spontaneous" tumor models. The effect appears to be due in part to PPARgamma-mediated downregulation of metallothioneins, proteins that have been shown to be involved in resistance to platinum-based therapy. These data strongly suggest combining PPARgamma agonists and platinum-based drugs for the treatment of certain human cancers.
Figures
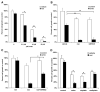
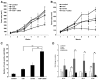
Figure 1. PPARγ Activation Enhances Carboplatin Growth Inhibition in A549 Lung Adenocarcinoma Cells
(A) A549 cells treated with 0.5 μM of rosiglitazone and indicated doses of carboplatin alone or in combination. (B) A549 cells were treated with 1 μm rosiglitazone, 250 nM GW1929, or carboplatin, alone or in combination. (C) A549 cells were treated with 0.5 μM rosiglitazone alone or in combination with 250 nM GW9662, with and without carboplatin. (D) Interaction of rosiglitazone with different platinum-based drugs. A549 cells treated with 0.5 μM rosiglitazone, 10 μM carboplatin, 1 μM cisplatin, or 1 μM oxaliplatin alone or in combination. Cell number was determined after 7–10 days and expressed as a percent of control cells. Representative experiments, n = 3, mean ± SD. *p < 0.05, **p < 0.01.
Figure 2. Rosiglitazone and Carboplatin Synergize to Suppress Growth in Multiple Cell Types
Cells were treated with the indicated concentrations of carboplatin and 0.5 μM rosiglitazone or (B) 0.2 μM rosiglitazone. (A and B) NSCLC adenocarcinoma, (C) squamous cell carcinoma, (D) adenosquamous cell carcinoma, (E) OVCA420, or (F) OVCA429 ovarian epithelial cancer cell lines were treated with 1 μM rosiglitazone and 10 μM carboplatin alone or in combination. Cell number was determined after 7–10 days and expressed as a percent of control cells. Representative experiments, n = 3 mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001.
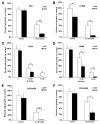
Figure 3. Combination Treatment Increases G2-M Arrest and Apoptosis
A549 cells were treated with 0.5 μM rosiglitazone or 10 μM carboplatin alone or in combination. (A) Cell-cycle analysis was determined by PI staining using FACS. n = 3 ± SD. (B) Immunoblotting for cleaved PARP-1. 115 kDa, uncleaved PARP1; 85 kDa, cleaved PARP1. (C) Percentage of apoptotic cells as determined by annexin V-positive cells using FACS analysis. Representative experiments, n = 3 mean ± SD. *p < 0.05, **p < 0.01.
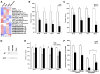
Figure 4. Rosiglitazone Suppresses Several Members of the Metallothionein Gene Family
(A) Heat diagram of cluster analysis for heavy metal binding proteins from microarray data of RNA following treatment with rosiglitazone, carboplatin, or a combination of the two. (B) Real-time PCR for the expression of metallothioneins 1G, 1H, 1X, and IIA following treatment of A549 cells with 1 μM rosiglitazone for 24 hr. (C) Real-time PCR for the expression of metallothioneins 1G, 1H, 1X, and IIA following treatment of A549 cells with 250 nM GW1929 for 24 hr. (D) Metallothionein protein expression in A549 cells following treatment with PPARγ agonists GW1929 (250 nM) or rosiglitazone (1 μM) for 24 hr. (E) A549 cells were treated with 1 μM rosiglitazone alone or in combination with the PPARγ antagonist GW9662 for 24 hr, and real-time PCR was carried out for MT1G, MT1H, MT1X, and MTIIA. *p < 0.05 rosi + GW9662 versus rosiglitazone alone. (F) Ectopic expression of MT1H blunts the synergistic effect of rosiglitazone and carboplatin. A549 cells stably transduced with a control or retrovirus expressing MT1H were treated with 0.5 μM rosiglitazone or 10 μM carboplatin alone or in combination, and the cell number was determined using a hemocytometer after 7 days. Representative experiment, n = 3 mean ± SD. *p < 0.05.
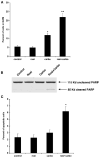
Figure 5. Rosiglitazone and Carboplatin Synergize In Vivo to Reduce Tumor Growth
1 × 107 A549 cells were injected subcutaneously into the flank of nude mice. Once the tumor reached 50–75 mm3, treatments were initiated. (A) Control chow, chow containing 5 mg/kg/day rosiglitazone, 10 mg/kg carboplatin IP two times per week or in combination. (B) Control chow, chow containing 20 mg/kg rosiglitazone/day, 50 mg/kg carboplatin IP two times per week or in combination. Tumor growth was measured two times per week. n = 10 mice per group, mean ± SD. *p < 0.05. (C) Average TUNEL-positive cells per field from paraffin-embedded sections of animals treated with either drug alone or in combination. n = 5–8 tumors per group, 4 fields per tumor section, mean ± SD. (D) PPARγ suppresses MT gene expression in vivo. RNA was isolated from tumors of mice treated with rosiglitazone and carboplatin and expression of the indicated metallothioneins determined. n = 6–9 tumors per group, mean ± SD. *p < 0.05, **p < 0.0005.
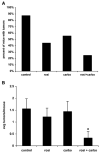
Figure 6. Rosiglitazone and Carboplatin Synergize In Vivo in a Spontaneous Tumor Model
Mice were treated with 10 mg/kg AOM once a week for 6 weeks. Following an additional 12 weeks, mice were treated as described in the Experimental Procedures for 6 weeks. Mice were then euthanized and examined for tumor incidence (A) or number of polyps per mouse (B). n = 8–9 mice per group, mean ± SD. *p < 0.05 versus control, rosiglitazone alone, or carboplatin alone.
Similar articles
- Regression of drug-resistant lung cancer by the combination of rosiglitazone and carboplatin.
Girnun GD, Chen L, Silvaggi J, Drapkin R, Chirieac LR, Padera RF, Upadhyay R, Vafai SB, Weissleder R, Mahmood U, Naseri E, Buckley S, Li D, Force J, McNamara K, Demetri G, Spiegelman BM, Wong KK. Girnun GD, et al. Clin Cancer Res. 2008 Oct 15;14(20):6478-86. doi: 10.1158/1078-0432.CCR-08-1128. Clin Cancer Res. 2008. PMID: 18927287 Free PMC article. - Ciglitazone, an agonist of peroxisome proliferator-activated receptor gamma, exerts potentiated cytostatic/cytotoxic effects against tumor cells when combined with lovastatin.
Mrówka P, Glodkowska E, Nowis D, Legat M, Issat T, Makowski M, Szokalska A, Janowska S, Stoklosa T, Jakóbisiak M, Golab J. Mrówka P, et al. Int J Oncol. 2008 Jan;32(1):249-55. Int J Oncol. 2008. PMID: 18097565 - Beyond peroxisome proliferator-activated receptor gamma signaling: the multi-facets of the antitumor effect of thiazolidinediones.
Weng JR, Chen CY, Pinzone JJ, Ringel MD, Chen CS. Weng JR, et al. Endocr Relat Cancer. 2006 Jun;13(2):401-13. doi: 10.1677/erc.1.01182. Endocr Relat Cancer. 2006. PMID: 16728570 Review. - [Anti-cancer action by PPARgamma ligand].
Okumura T. Okumura T. Nihon Rinsho. 2010 Feb;68(2):267-72. Nihon Rinsho. 2010. PMID: 20158095 Review. Japanese.
Cited by
- Molecular mechanisms of cancer development in obesity.
Khandekar MJ, Cohen P, Spiegelman BM. Khandekar MJ, et al. Nat Rev Cancer. 2011 Nov 24;11(12):886-95. doi: 10.1038/nrc3174. Nat Rev Cancer. 2011. PMID: 22113164 Review. - Epigenetic derepression converts PPARγ into a druggable target in triple-negative and endocrine-resistant breast cancers.
Loo SY, Syn NL, Koh AP, Teng JC, Deivasigamani A, Tan TZ, Thike AA, Vali S, Kapoor S, Wang X, Wang JW, Tan PH, Yip GW, Sethi G, Huang RY, Hui KM, Wang L, Goh BC, Kumar AP. Loo SY, et al. Cell Death Discov. 2021 Sep 27;7(1):265. doi: 10.1038/s41420-021-00635-5. Cell Death Discov. 2021. PMID: 34580286 Free PMC article. - MKP1 mediates chemosensitizer effects of E1a in response to cisplatin in non-small cell lung carcinoma cells.
Cimas FJ, Callejas-Valera JL, Pascual-Serra R, García-Cano J, Garcia-Gil E, De la Cruz-Morcillo MA, Ortega-Muelas M, Serrano-Oviedo L, Gutkind JS, Sánchez-Prieto R. Cimas FJ, et al. Oncotarget. 2015 Dec 29;6(42):44095-107. doi: 10.18632/oncotarget.6574. Oncotarget. 2015. PMID: 26689986 Free PMC article. - Associations between body mass index, weight loss and overall survival in patients with advanced lung cancer.
Oswalt C, Liu Y, Pang H, Le-Rademacher J, Wang X, Crawford J. Oswalt C, et al. J Cachexia Sarcopenia Muscle. 2022 Dec;13(6):2650-2660. doi: 10.1002/jcsm.13095. Epub 2022 Oct 20. J Cachexia Sarcopenia Muscle. 2022. PMID: 36268548 Free PMC article. - Role of the autotaxin-lysophosphatidate axis in cancer resistance to chemotherapy and radiotherapy.
Brindley DN, Lin FT, Tigyi GJ. Brindley DN, et al. Biochim Biophys Acta. 2013 Jan;1831(1):74-85. doi: 10.1016/j.bbalip.2012.08.015. Epub 2012 Aug 29. Biochim Biophys Acta. 2013. PMID: 22954454 Free PMC article. Review.
References
- ACS. Cancer Facts and Figures. Atlanta: American Cancer Society; 2006.
- Akiyama S, Chen ZS, Sumizawa T, Furukawa T. Resistance to cisplatin. Anticancer Drug Des. 1999;14:143–151. - PubMed
- Andrews PA, Murphy MP, Howell SB. Metallothionein-mediated cisplatin resistance in human ovarian carcinoma cells. Cancer Chemother Pharmacol. 1987;19:149–154. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 DK031405-25/DK/NIDDK NIH HHS/United States
- R01 DK057670-05/DK/NIDDK NIH HHS/United States
- K01 DK064685/DK/NIDDK NIH HHS/United States
- K01 DK064685-04/DK/NIDDK NIH HHS/United States
- R37 DK 31405/DK/NIDDK NIH HHS/United States
- R01 DK 57670/DK/NIDDK NIH HHS/United States
- DK 064685/DK/NIDDK NIH HHS/United States
- R01 DK057670/DK/NIDDK NIH HHS/United States
- R37 DK031405/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases