Mechanism and uses of a membrane peptide that targets tumors and other acidic tissues in vivo - PubMed (original) (raw)
Mechanism and uses of a membrane peptide that targets tumors and other acidic tissues in vivo
Oleg A Andreev et al. Proc Natl Acad Sci U S A. 2007.
Abstract
The pH-selective insertion and folding of a membrane peptide, pHLIP [pH (low) insertion peptide], can be used to target acidic tissue in vivo, including acidic foci in tumors, kidneys, and inflammatory sites. In a mouse breast adenocarcinoma model, fluorescently labeled pHLIP finds solid acidic tumors with high accuracy and accumulates in them even at a very early stage of tumor development. The fluorescence signal is stable for >4 days and is approximately five times higher in tumors than in healthy counterpart tissue. In a rat antigen-induced arthritis model, pHLIP preferentially accumulates in inflammatory foci. pHLIP also maps the renal cortical interstitium; however, kidney accumulation can be reduced significantly by providing mice with bicarbonate-containing drinking water. The peptide has three states: soluble in water, bound to the surface of a membrane, and inserted across the membrane as an alpha-helix. At physiological pH, the equilibrium is toward water, which explains its low affinity for cells in healthy tissue; at acidic pH, titration of Asp residues shifts the equilibrium toward membrane insertion and tissue accumulation. The replacement of two key Asp residues located in the transmembrane part of pHLIP by Lys or Asn led to the loss of pH-sensitive insertion into membranes of liposomes, red blood cells, and cancer cells in vivo, as well as to the loss of specific accumulation in tumors. pHLIP nanotechnology introduces a new method of detecting, targeting, and possibly treating acidic diseased tissue by using the selective insertion and folding of membrane peptides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
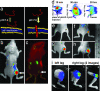
Imaging tumors and inflammation. (a) The mechanism of pHLIP interaction with lipid bilayers. The peptide has three states: soluble in water, bound to the surface of a membrane (at normal pH 7.4), and inserted across the membrane as an α-helix (at low pH). (b) Overlay of pHLIP-Cy5.5 fluorescence and light images of mice bearing a tumor (7 mm in diameter, 12 d after 106 cell implant) in the right flank obtained on the homemade imager (i.p. injection of 500 μg/kg of pHLIP-Cy5.5 1 d before imaging). (c) pHLIP-Alexa750 fluorescent image (excitation 750 nm, emission 800 nm, artificial green color) of mice bearing a tumor (2 mm in diameter 6 d after 106 cell implant) in the right flank obtained on the IR scanner with focal distance set at 3 mm, which allows for the collection of light from the interior of the body (i.p. injection of 300 μg/kg of pHLIP-Alexa750 1 d before imaging). Reflectance is denoted in red (excitation 680 nm). (d) pHLIP-Cy5.5 given as a single i.p. injection (200 μg/kg) into the left side of mice initially diffused into the left flank, but 20 h later it accumulated in a tumor on the right flank. The fluorescent image of the back part of each mouse is presented. Blue color represents the background fluorescent signal, and the red color represents a high intensity of the fluorescence signal. (e–h) Overlay of pHLIP-Cy5.5 (500 μg/kg) fluorescence and light images of back part of mice bearing tumors of different sizes in right flanks: (e) undetectable by eye at time of imaging 5 d after 105 cell implant, (f) 3 × 4 mm (8 d after 106 cell implant), (g) 5 × 6 mm (12 d after 106 cell implant), (h) 8 × 9 mm (18 d after 106 cell implant). (i) Accumulation of pHLIP- Cy5.5 (2 days after i.p injection, 30 μg/kg) in inflammation sites is shown by overlay of pHLIP fluorescence and photo images of rat right (arthritis) and left (control) legs. The arthritis was induced in the right leg by injection of methylated BSA and Freund's complete adjuvant (the left knee of the rat received a sham injection of saline and was used as a control). A substantial fluorescence signal (4–5 times higher than in the left knee joint) was detected in the right knee (1), especially in the knee joint (2) (red color represents high fluorescence intensity).
Fig. 2.
Interaction of pHLIP and its variants with membranes of liposomes, human RBCs, and cancer cells in vivo. Trp fluorescence (a) and CD (b) spectra of pHLIP [consisting of
l
–amino acids (solid lines) and
d
-amino acids (dotted lines)], K-pHLIP, and N-pHLIP were recorded to monitor the process of their interaction with 1-palmitoyl-2-oleoyl-_sn_-glycero-3-phosphocholine liposomes at normal and low pH. The fluorescence excitation wavelength was 295 nm for the excitation of Trp fluorophores. The concentrations of peptides and lipids were 5 and 600 μM, respectively. Black signifies fluorescence and CD spectra of pHLIP at pH 8.0 in the absence of liposomes; blue, after 30 min of incubation with liposomes at pH 8.0; red, after incubation with liposomes at pH 4.5. (c) Binding of various agents selectively to the outer leaflet of RBCs can change the biconcave discoid shape of the cells (left image) into convex structures on the cell surface (right image), echinocytic spicules. (d) Phase-contrast images of 1% suspensions of human RBCs incubated for 15 min with 5 μM pHLIP, K-pHLIP, and N-pHLIP at pH 7.4 and 6.0 are presented. RBCs without peptides had normal discoid shape at both pH 7.4 and 6.0 (images are not shown). Statistical data (percentage of cells having spicules) are shown in Table 1. To avoid induction of echinocytosis by proximity to a glass surface, glass slides were treated with 2% dimethyldichlorosilane in 1,1,1-trichloromethane for 10 min and rinsed with methanol, ethanol, and water and allowed to dry before use in experiments. Grace Bio-Labs (Bend, OR) CoverWell imaging chambers were used for observation of RBC/peptide suspensions instead of glass coverslips. The pictures were taken on an Olympus IX71 inverted-fluorescence microscope with an ×100 objective. (e) Overlay of NIR fluorescence and x-ray images obtained by using a Kodak In-Vivo FX image station on the next day after i.p. injection of 500 μg/kg pHLIP-Cy5.5, K-pHLIP-Cy5.5, N-pHLIP-Cy5.5, or molar equivalent amount of Cy5.5 alone on mice bearing tumors in the right flank. The injection of peptide was performed when the tumor was not detectable by eye on the sixth day after cancer-cell implantation (5 × 104 cells); imaging was performed on the next day. Tumors became visible 2 weeks later. Replacement of two key Asp residues located in the transmembrane part of pHLIP by Lys or Asn leads to the loss of pH-sensitive insertion into membranes of liposomes, RBCs, and cancer cells in vivo, as well as to the loss of specific accumulation in tumors.
Fig. 3.
CI and distribution of pHLIP in organs. (a) The 3D presentation of NIR fluorescence images of mice bearing a tumor (4 × 5 mm size) 8 days after i.p. injection of 500 μg/kg of pHLIP-Cy5.5, which was done on the seventh day of tumor growth. The height (z axes) and intensity of red color indicate the strength of the NIR signal in the right flank of a mouse where the tumor was implanted. (b) NIR fluorescence and light images of kidneys ( a and b), tumor (2), skin (3), stomach cleaned and separated into 2 parts (4), liver (5), muscle (6), lung (7), spleen (8), heart (9), and brain (10) collected from a mouse bearing a tumor on the next day after injection of 500 μg/kg of pHLIP-Cy5.5 consisting of
l
-amino acids or
d
-amino acids. (c) The CI, which indicates the relative strength of the fluorescence signal in tumors, was calculated as a ratio: CI = [(_Fl_tumor − _Fl_auto)/(_Fl_norm − _Fl_auto)]. _Fl_tumor and _Fl_norm are the mean fluorescence intensities of tumor and normal contralateral regions of the same area, respectively, and _Fl_auto is the autofluorescence from the corresponding region measured before injection. The fluorescence signal from the tumor was approximately five times stronger in comparison with the contralateral region. The CI was calculated for >20 animals. (d) The fluorescence signals were measured from organs collected the next day after i.p. injection of 500 μg/kg of pHLIP-Cy5.5 consisting of
l
-amino acids and
d
-amino acids. The signals were normalized to the intensity of kidney fluorescence. (e) The NIR fluorescence of kidney 2 days after i.p. injection of 500 μg/kg of pHLIP-Alexa750 into mice fed with regular water (Left) and 80 mM NaHCO3 (pH 8.2) solution (Right) obtained on the IR scanner. The alkalinization resulted in an ≈50% reduction of pHLIP accumulation in the kidney.
Similar articles
- Cy5.5-labeled pH low insertion peptide (pHLIP).
Shan L. Shan L. 2009 Aug 8 [updated 2009 Nov 12]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2009 Aug 8 [updated 2009 Nov 12]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641819 Free Books & Documents. Review. - Energetics of peptide (pHLIP) binding to and folding across a lipid bilayer membrane.
Reshetnyak YK, Andreev OA, Segala M, Markin VS, Engelman DM. Reshetnyak YK, et al. Proc Natl Acad Sci U S A. 2008 Oct 7;105(40):15340-5. doi: 10.1073/pnas.0804746105. Epub 2008 Sep 30. Proc Natl Acad Sci U S A. 2008. PMID: 18829441 Free PMC article. - 64Cu-1,4,7,10-Tetraazacyclododecane-1,4,7-Tris-acetic acid-10-maleimidoethylacetamide-ACEQNPIYWARYADWLFTTPLLLLDLALLVDADEGTG.
Shan L. Shan L. 2009 May 29 [updated 2009 Jul 27]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2009 May 29 [updated 2009 Jul 27]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641332 Free Books & Documents. Review. - Membrane-Induced p Ka Shifts in wt-pHLIP and Its L16H Variant.
Vila-Viçosa D, Silva TFD, Slaybaugh G, Reshetnyak YK, Andreev OA, Machuqueiro M. Vila-Viçosa D, et al. J Chem Theory Comput. 2018 Jun 12;14(6):3289-3297. doi: 10.1021/acs.jctc.8b00102. Epub 2018 May 17. J Chem Theory Comput. 2018. PMID: 29733633 Free PMC article. - Roles of carboxyl groups in the transmembrane insertion of peptides.
Barrera FN, Weerakkody D, Anderson M, Andreev OA, Reshetnyak YK, Engelman DM. Barrera FN, et al. J Mol Biol. 2011 Oct 21;413(2):359-71. doi: 10.1016/j.jmb.2011.08.010. Epub 2011 Aug 23. J Mol Biol. 2011. PMID: 21888917 Free PMC article.
Cited by
- A pH-dependent charge reversal peptide for cancer targeting.
Wakabayashi N, Yano Y, Kawano K, Matsuzaki K. Wakabayashi N, et al. Eur Biophys J. 2017 Mar;46(2):121-127. doi: 10.1007/s00249-016-1145-y. Epub 2016 Jun 8. Eur Biophys J. 2017. PMID: 27278924 - Antiproliferative effect of pHLIP-amanitin.
Moshnikova A, Moshnikova V, Andreev OA, Reshetnyak YK. Moshnikova A, et al. Biochemistry. 2013 Feb 19;52(7):1171-8. doi: 10.1021/bi301647y. Epub 2013 Feb 8. Biochemistry. 2013. PMID: 23360641 Free PMC article. - pHLIP-mediated translocation of membrane-impermeable molecules into cells.
Thévenin D, An M, Engelman DM. Thévenin D, et al. Chem Biol. 2009 Jul 31;16(7):754-62. doi: 10.1016/j.chembiol.2009.06.006. Chem Biol. 2009. PMID: 19635412 Free PMC article. - Determination of the Membrane Translocation pK of the pH-Low Insertion Peptide.
Scott HL, Westerfield JM, Barrera FN. Scott HL, et al. Biophys J. 2017 Aug 22;113(4):869-879. doi: 10.1016/j.bpj.2017.06.065. Biophys J. 2017. PMID: 28834723 Free PMC article. - Family of pH (low) insertion peptides for tumor targeting.
Weerakkody D, Moshnikova A, Thakur MS, Moshnikova V, Daniels J, Engelman DM, Andreev OA, Reshetnyak YK. Weerakkody D, et al. Proc Natl Acad Sci U S A. 2013 Apr 9;110(15):5834-9. doi: 10.1073/pnas.1303708110. Epub 2013 Mar 25. Proc Natl Acad Sci U S A. 2013. PMID: 23530249 Free PMC article.
References
- Stubbs M, McSheehy PMJ, Griffiths JR, Bashford CL. Mol Med Today. 2000;6:15–19. - PubMed
- Naghavi M, John R, Naguib S, Siadaty MS, Grasu R, Kurian KC, van Winkle WB, Soller B, Litovsky S, Madjid M, et al. Atherosclerosis. 2002;164:27–35. - PubMed
- Izumi H, Torigoe T, Ishiguchi H, Uramoto H, Yoshida Y, Tanabe M, Ise T, Murakami T, Yoshida T, Nomoto M, et al. Cancer Treat Rev. 2003;29:541–549. - PubMed
- Huang Y, McNamara JO. Cell. 2004;118:665–666. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM070895/GM/NIGMS NIH HHS/United States
- P20 RR016457/RR/NCRR NIH HHS/United States
- GM073857/GM/NIGMS NIH HHS/United States
- P20RR016457/RR/NCRR NIH HHS/United States
- GM070895/GM/NIGMS NIH HHS/United States
- R01 GM073857/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources