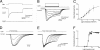High-speed mapping of synaptic connectivity using photostimulation in Channelrhodopsin-2 transgenic mice - PubMed (original) (raw)
. 2007 May 8;104(19):8143-8.
doi: 10.1073/pnas.0700384104. Epub 2007 May 1.
J Peca, M Matsuzaki, K Matsuzaki, J Noguchi, L Qiu, D Wang, F Zhang, E Boyden, K Deisseroth, H Kasai, W C Hall, G Feng, G J Augustine
Affiliations
- PMID: 17483470
- PMCID: PMC1876585
- DOI: 10.1073/pnas.0700384104
High-speed mapping of synaptic connectivity using photostimulation in Channelrhodopsin-2 transgenic mice
H Wang et al. Proc Natl Acad Sci U S A. 2007.
Abstract
To permit rapid optical control of brain activity, we have engineered multiple lines of transgenic mice that express the light-activated cation channel Channelrhodopsin-2 (ChR2) in subsets of neurons. Illumination of ChR2-positive neurons in brain slices produced photocurrents that generated action potentials within milliseconds and with precisely timed latencies. The number of light-evoked action potentials could be controlled by varying either the amplitude or duration of illumination. Furthermore, the frequency of light-evoked action potentials could be precisely controlled up to 30 Hz. Photostimulation also could evoke synaptic transmission between neurons, and, by scanning with a small laser light spot, we were able to map the spatial distribution of synaptic circuits connecting neurons within living cerebral cortex. We conclude that ChR2 is a genetically based photostimulation technology that permits analysis of neural circuits with high spatial and temporal resolution in transgenic mammals.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
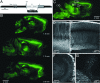
Transgenic expression of Chr2-YFP in mouse brain. (A) Thy1-Chr2-YFP construct. cDNA encoding a Chr2-YFP fusion protein was placed under the control of the regulatory elements of the mouse Thy1.2 gene. (B) Serial parasagittal sections from an adult transgenic mouse brain (line 9). Distance from the midline is indicated below each YFP fluorescence image. (C) Expression of Chr2-YFP in various regions of an adult line 18 mouse brain. Rectangles indicate areas enlarged in D–G below. (D) In the cortex, layer V neurons express high levels of Chr2-YFP and their apical dendrites are clearly labeled. (E and F) In the hippocampus both CA1 and CA3 neurons express ChR2-YFP. (G) Cerebellar mossy fibers show high levels of Chr2-YFP expression.
Fig. 2.
Illumination evokes photocurrents in ChR2-positive cortical neurons. (A) Prolonged light flash (top trace) generated a photocurrent (middle trace) in a neuron that expressed ChR2. No photocurrent was observed in a ChR2-negative neuron from a wild-type mouse (bottom trace). (B) The peak amplitude of photocurrents (lower trace) increased as a function of light intensity (upper trace). (C) Relationship between photocurrent amplitude and light intensity (100-ms duration); points indicate means ± SEM for seven neurons. Curve is a fit of the Hill equation. (D and E) Photocurrent (bottom traces) varied with light duration (top traces). (D) Responses to brief light pulses (1- to 8-ms durations). (E) Responses to longer duration flashes (10- to 100-ms durations). (F) Relationship between photocurrent amplitude and photostimulus duration (9.2 mW/mm2 luminance). Curve is fit of the Hill equation.
Fig. 3.
Illumination controls number and frequency of action potentials. (A) Light of varying luminance elicited graded changes in membrane potential and varying numbers of action potentials. (B) Relationship between light intensity and number of light-evoked action potentials (n = 4). Curve is fit of the Hill equation. (C) Varying the frequency of brief light flashes (4 ms duration; 9.2 mW/mm2) caused proportional changes in action potential frequency. (D) Mean probability of evoked action potentials decreased with light pulse frequency (n = 4). Curve is a Lorentzian function, with a roll-off frequency of 35 Hz.
Fig. 4.
Mapping light sensitivity of pyramidal neuron. (A) Structure of a pyramidal neuron filled with Alexa 594 dye that was dialyzed from a patch pipette (to the right). Here, and in subsequent figures, the top of the image is closest to the pial surface. Circles indicate locations where photoresponses in B were evoked. (B) Changes in membrane potential evoked by light spots (488 nm; 48 mW/mm2) positioned at the sites indicated in A. Only illumination near the cell body evoked an action potential (trace 3). Bar indicates time of illumination. (C) Scanning the light spot across the specimen revealed locations where light-induced depolarizations were evoked; pseudocolor scale at right indicates the amplitude of these responses. Red reflects action potentials.
Fig. 5.
Mapping of local excitatory circuits innervating cortical interneurons. (A) Image of ChR2-YFP fluorescence in layer VI of a living cortical slice. Bright structure on right side of image is thread used to anchor slice. (B) Dye-filled interneuron, with circles indicating locations where photoresponses in C were evoked. (C) Light-induced postsynaptic currents (holding potential = 70 mV), detected when a light spot (488 nm; 24 mW/mm2) was positioned at the locations indicated in B. (D) Map of spatial distribution of locations where light evoked synaptic currents; the magnitude of these currents is indicated by the pseudocolor scale at right. (E) Light-evoked currents (Left) were blocked by coapplication of 10 μM CNQX and 50 μM picrotoxin (PTX) (Right), indicating that they resulted from synaptic activity. Bars in C and E indicate time of illumination.
Fig. 6.
Properties of cortical microcircuits. (A) Fluorescence image of dye-filled layer VI pyramidal neuron. (B) Map of synaptic circuit innervating the pyramidal neuron shown in A. (C) Traces indicate excitatory postsynaptic currents (holding potential = −70 mV) measured when the light spot was positioned at the indicated locations in A. (D) Fluorescence image of dye-filled layer VI pyramidal neuron; circles indicate locations where light-evoked synaptic responses shown at the bottom of E were evoked. (E) Map of excitatory synaptic inputs innervating the neuron shown in D. Traces below the map indicate excitatory postsynaptic currents, measured at a holding potential of −70 mV, when the light spot was positioned at the locations indicated in D. (F) Treatment with 10 μM CNQX almost completely blocked the responses measured at −70 mV (compare with E), indicating that the responses in E are largely glutamatergic excitatory postsynaptic currents. (G) Same conditions as in (E) except that the postsynaptic membrane potential was changed to −40 mV, to reveal outward IPSCs (traces below). Comparison of E with G shows that inhibitory and excitatory inputs have different spatial distributions on the same cell. Bars in C and E–G indicate time of illumination.
Similar articles
- Channelrhodopsin as a tool to investigate synaptic transmission and plasticity.
Schoenenberger P, Schärer YP, Oertner TG. Schoenenberger P, et al. Exp Physiol. 2011 Jan;96(1):34-9. doi: 10.1113/expphysiol.2009.051219. Epub 2010 Jun 18. Exp Physiol. 2011. PMID: 20562296 Review. - Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections.
Petreanu L, Huber D, Sobczyk A, Svoboda K. Petreanu L, et al. Nat Neurosci. 2007 May;10(5):663-8. doi: 10.1038/nn1891. Epub 2007 Apr 15. Nat Neurosci. 2007. PMID: 17435752 - Spatio-temporal control of neural activity in vivo using fluorescence microendoscopy.
Hayashi Y, Tagawa Y, Yawata S, Nakanishi S, Funabiki K. Hayashi Y, et al. Eur J Neurosci. 2012 Sep;36(6):2722-32. doi: 10.1111/j.1460-9568.2012.08191.x. Epub 2012 Jul 11. Eur J Neurosci. 2012. PMID: 22780218 - New optical tools for controlling neuronal activity.
Herlitze S, Landmesser LT. Herlitze S, et al. Curr Opin Neurobiol. 2007 Feb;17(1):87-94. doi: 10.1016/j.conb.2006.12.002. Epub 2006 Dec 15. Curr Opin Neurobiol. 2007. PMID: 17174547 Review. - An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology.
Aravanis AM, Wang LP, Zhang F, Meltzer LA, Mogri MZ, Schneider MB, Deisseroth K. Aravanis AM, et al. J Neural Eng. 2007 Sep;4(3):S143-56. doi: 10.1088/1741-2560/4/3/S02. Epub 2007 May 31. J Neural Eng. 2007. PMID: 17873414
Cited by
- Synaptic cooperativity regulates persistent network activity in neocortex.
Favero M, Castro-Alamancos MA. Favero M, et al. J Neurosci. 2013 Feb 13;33(7):3151-63. doi: 10.1523/JNEUROSCI.4424-12.2013. J Neurosci. 2013. PMID: 23407969 Free PMC article. - Spatial extent of cochlear infrared neural stimulation determined by tone-on-light masking.
Matic AI, Walsh JT Jr, Richter CP. Matic AI, et al. J Biomed Opt. 2011 Nov;16(11):118002. doi: 10.1117/1.3655590. J Biomed Opt. 2011. PMID: 22112140 Free PMC article. - Input- and cell-type-specific endocannabinoid-dependent LTD in the striatum.
Wu YW, Kim JI, Tawfik VL, Lalchandani RR, Scherrer G, Ding JB. Wu YW, et al. Cell Rep. 2015 Jan 6;10(1):75-87. doi: 10.1016/j.celrep.2014.12.005. Epub 2014 Dec 24. Cell Rep. 2015. PMID: 25543142 Free PMC article. - Neural and hemodynamic responses elicited by forelimb- and photo-stimulation in channelrhodopsin-2 mice: insights into the hemodynamic point spread function.
Vazquez AL, Fukuda M, Crowley JC, Kim SG. Vazquez AL, et al. Cereb Cortex. 2014 Nov;24(11):2908-19. doi: 10.1093/cercor/bht147. Epub 2013 Jun 12. Cereb Cortex. 2014. PMID: 23761666 Free PMC article. - Task Learning Promotes Plasticity of Interneuron Connectivity Maps in the Olfactory Bulb.
Huang L, Ung K, Garcia I, Quast KB, Cordiner K, Saggau P, Arenkiel BR. Huang L, et al. J Neurosci. 2016 Aug 24;36(34):8856-71. doi: 10.1523/JNEUROSCI.0794-16.2016. J Neurosci. 2016. PMID: 27559168 Free PMC article.
References
- Pettit DL, Helms MC, Lee P, Augustine GJ, Hall WC. J Neurophysiol. 1999;81:1424–1427. - PubMed
- Lima SQ, Miesenbock G. Cell. 2005;121:141–152. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases