Oncogenic activation of c-Abl in non-small cell lung cancer cells lacking FUS1 expression: inhibition of c-Abl by the tumor suppressor gene product Fus1 - PubMed (original) (raw)
Oncogenic activation of c-Abl in non-small cell lung cancer cells lacking FUS1 expression: inhibition of c-Abl by the tumor suppressor gene product Fus1
J Lin et al. Oncogene. 2007.
Abstract
In lung cancer, frequent loss of one allele of chromosome 3p is seen in both small cell lung cancer and non-small cell lung cancer (NSCLC), providing evidence of tumor suppressor genes (TSGs) in this chromosomal region. The mechanism of Fus1 tumor suppressor activity is unknown. We have found that a Fus1 peptide inhibits the Abl tyrosine kinase in vitro (IC(50) 35 microM). The inhibitory Fus1 sequence was derived from a region that was deleted in a mutant FUS1 gene (FUS1 (1-80)) detected in some lung cancer cell lines. Importantly, a stearic acid-modified form of this peptide was required for the inhibition, but stearic acid alone was not inhibitory. Two NSCLC cell lines, which lack expression of wild-type Fus1, contain activated c-Abl. Forced expression of an inducible FUS1 cDNA in H1299 NSCLC cells decreased levels of activated c-Abl and inhibited its tyrosine kinase activity. Similarly, treatment of c-Abl immune complexes with the inhibitory Fus1 peptide also reduced the level of c-Abl in these immune complexes. The size and number of colonies of the NSCLC cell line, H1,299, in soft agar was strongly inhibited by the Abl kinase inhibitor imatinib mesylate. Co-expression of FUS1 and c-ABL in COS1 cells blocked activation of c-Abl tyrosine kinase. In contrast, co-expression of mutant FUS1 (1-80) with c-ABL had little inhibitory activity against c-Abl. These findings provide strong evidence that c-Abl is a possible target in NSCLC patients that have reduced expression of Fus1 in their tumor cells.
Figures
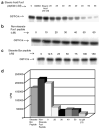
Figure 1. Stearic acid-modified Fus1 peptide inhibits constitutively activated commercial Abl tyrosine kinase
(a) Stearic acid-modified Fus1 peptide inhibits GST–Crk phosphorylation by a bacterially purified, constitutively active, Abl kinase (IC50 35uM). (b) Without the stearic acid modification, the Fus1 peptide does not inhibit GST–Crk phosphorylation by the bacterially purified, constitutively active, Abl kinase. (c) A stearic acid-modified Bcr peptide does not inhibit GST–Crk phosphorylation by the bacterially purified, constitutively active, Abl kinase. (d) Stearate Fus1 peptide inhibits phosphorylation of a known Abl kinase peptide target, EAIYAAPFAKKK, by full-length human Abl.
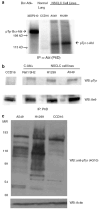
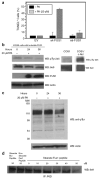
Figure 2. Detection of activated c-Abl tyrosine kinase in two NSCLC cell lines deficient of FUS1
(a) NSCLC cell lines A549 and H1299 contain an active c-Abl tyrosine kinase, as measured in an in vitro kinase assay. Cells were lysed and incubated with the monoclonal anti-Abl antibody P6D, raised against c-Abl 51–64 residues (Liu et al., 1993). The immune complex was pulled down using protein A beads and subjected to either kinase assays or western blotting. 32D cells expressing Bcr-Abl were used as a positive control. Normal human lung fibroblast, CCD16 and 32D cells expressing Bcr–Abl were used as negative and positive controls, respectively. (b) Anti-phosphotyrosine western blotting demonstrates that NSCLC cell lines A549 and H1299 have an active c-Abl tyrosine kinase while normal human lung fibroblast CCD16 lack an active c-Abl tyrosine kinase. Phosphotyrosine c-Abl was detected by anti-phosphotyrosine antibody 4G10 and c-Abl was detected by monoclonal anti-Abl antibody 8e9. Transformed Rat 1cells (Rat1 SH2) that express activated c-Abl was used a positive control (Ling et al., 2003). (c) NSCLC cell lines have increased levels of phosphotyrosine-containing proteins compared to normal lung fibroblast cells (CCD16) as measured by 4G10.
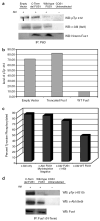
Figure 3. Wild-type full-length Fus1 down regulates c-Abl tyrosine kinase
(a) c-ABL was co-transfected with either wild-type full-length FUS1, truncated FUS1, or empty vector in COS1 cells. At 48 h post-transfection, COS1 cells were harvested and lysate was incubated with the monoclonal anti-Abl antibody P6D. The immune complex was pulled down using protein A beads and subjected to western blotting. Antibody specific for pTyr412 of Abl (Abcam) was used to show kinase active c-Abl. The same blot was stripped and re-probed with monoclonal anti-Abl antibody 8e9. N-terminal Fus1 antibody was used to detect Fus1. (b) The intensities of the c-Abl pTyr 412 bands were normalized against c-Abl expression. (c) C-ABL was co-transfected with either wild-type full-length FUS1, truncated FUS1 or a non-myristoylated mutant FUS1 (Gly2Ala) in COS1 cells. At 48 h post-transfection, COS1 cells were harvested and the lysate was incubated with the monoclonal anti-Abl antibody P6D. The immune complex was pulled down using protein A beads and subjected to western blotting. Phosphotyrosine c-Abl was detected by anti-phosphotyrosine antibody 4G10 and the same blot was stripped and re-probed with monoclonal anti-Abl antibody 8e9 . The band intensities were normalized for c-Abl expression. (d) C-ABL was co-transfected with either wild-type full-length FUS1 or truncated FUS1 in COS1 cells. At 48 h post-transfection, COS1 cells were harvested and lysate was incubated with a polyclonal N-terminal Fus1 antibody. The immune complex was pulled down using protein A beads and subjected to western blotting. Phosphotyrosine c-Abl was detected by anti-phosphotyrosine antibody 4G10 and the same blot was stripped and re-probed with monoclonal anti-Abl antibody 8e9.
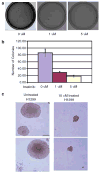
Figure 4. Imatinib decreases colony formation of NSCLC cell line H1299 in soft agar
(a) A total of 1000 H1299 cells were plated in 0.35% soft agar containing either 0, 1, or 5 _μ_M imatinib. Agar cultures were grown for 18 days changing the media every 2 days containing the respective concentrations of imatinib. (b) Dose-dependent inhibition of H1299 colony formation in soft agar by imatinib. (c) A total of 1000 H1299 cells were plated in 0.35% soft agar either containing 10 _μ_M imatinib or untreated. Cells were grown for 18 days changing the media every 2 days containing the respective concentrations of imatinib.
Figure 5. Induction of Fus1 in NSCLC H1299 cells induces cell death and decreases c-Abl kinase activity
(a) Induction of FUS1 expression in H1299 NSCLC cells induces apoptosis. Stable clones of human NSCLC H1299 that express an inducible wild-type FUS1 (wt-FUS1) or myristoylation mutant FUS1 (mt-FUS1) were treated with of 20 _μ_M of PA for 48 h. Apoptosis was measured by flow cytometry using a terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL)-based fluorescence-activated cell sorter (FACS) analysis. DNA fragmentation was analysed by FACS. The relative apoptotic cells were calculated as the percentage of the TUNEL-positive cells in the total cell populations. (b) Induction of FUS1 expression inactivated c-Abl tyrosine kinase and reduced the level of the c-Abl protein. H1299 cells with inducible FUS1 were treated with PA for 24 and 36 h. Whole cell lysate was subjected to western blot and the membrane was probed with c-Abl pTyr 245 antibody (Abcam), c-Abl antibody 8e9 and N-terminal Fus1 antibody. COS1 cells transfected with c-Abl were used as a positive control. (c) FUS1 expression in H1299 cells reduces the level of phosphotyrosine-containing proteins. (d) Fus1 peptide decreases the level of the c-Abl protein in immune complexes of c-Abl from COS1. Immune complexes were treated with indicated amount of Fus1 peptide for 20 min at 30°C.
Similar articles
- Involvement of Jak2 tyrosine phosphorylation in Bcr-Abl transformation.
Xie S, Wang Y, Liu J, Sun T, Wilson MB, Smithgall TE, Arlinghaus RB. Xie S, et al. Oncogene. 2001 Sep 27;20(43):6188-95. doi: 10.1038/sj.onc.1204834. Oncogene. 2001. PMID: 11593427 - Growth inhibition and modulation of kinase pathways of small cell lung cancer cell lines by the novel tyrosine kinase inhibitor STI 571.
Wang WL, Healy ME, Sattler M, Verma S, Lin J, Maulik G, Stiles CD, Griffin JD, Johnson BE, Salgia R. Wang WL, et al. Oncogene. 2000 Jul 20;19(31):3521-8. doi: 10.1038/sj.onc.1203698. Oncogene. 2000. PMID: 10918610 - Tumor suppressor FUS1 signaling pathway.
Ji L, Roth JA. Ji L, et al. J Thorac Oncol. 2008 Apr;3(4):327-30. doi: 10.1097/JTO.0b013e31816bce65. J Thorac Oncol. 2008. PMID: 18379348 Free PMC article. Review. - Mitochondrial Fus1/Tusc2 and cellular Ca2+ homeostasis: tumor suppressor, anti-inflammatory and anti-aging implications.
Uzhachenko R, Shimamoto A, Chirwa SS, Ivanov SV, Ivanova AV, Shanker A. Uzhachenko R, et al. Cancer Gene Ther. 2022 Oct;29(10):1307-1320. doi: 10.1038/s41417-022-00434-9. Epub 2022 Feb 18. Cancer Gene Ther. 2022. PMID: 35181743 Free PMC article. Review.
Cited by
- Activation of abl family kinases in solid tumors.
Ganguly SS, Plattner R. Ganguly SS, et al. Genes Cancer. 2012 May;3(5-6):414-25. doi: 10.1177/1947601912458586. Genes Cancer. 2012. PMID: 23226579 Free PMC article. - Exogenous Restoration of TUSC2 Expression Induces Responsiveness to Erlotinib in Wildtype Epidermal Growth Factor Receptor (EGFR) Lung Cancer Cells through Context Specific Pathways Resulting in Enhanced Therapeutic Efficacy.
Dai B, Yan S, Lara-Guerra H, Kawashima H, Sakai R, Jayachandran G, Majidi M, Mehran R, Wang J, Bekele BN, Baladandayuthapani V, Yoo SY, Wang Y, Ying J, Meng F, Ji L, Roth JA. Dai B, et al. PLoS One. 2015 Jun 8;10(6):e0123967. doi: 10.1371/journal.pone.0123967. eCollection 2015. PLoS One. 2015. PMID: 26053020 Free PMC article. - Genome-wide copy number variation pattern analysis and a classification signature for non-small cell lung cancer.
Qiu ZW, Bi JH, Gazdar AF, Song K. Qiu ZW, et al. Genes Chromosomes Cancer. 2017 Jul;56(7):559-569. doi: 10.1002/gcc.22460. Epub 2017 May 4. Genes Chromosomes Cancer. 2017. PMID: 28379620 Free PMC article. - Reciprocal regulation of Abl and receptor tyrosine kinases.
Srinivasan D, Kaetzel DM, Plattner R. Srinivasan D, et al. Cell Signal. 2009 Jul;21(7):1143-50. doi: 10.1016/j.cellsig.2009.03.003. Epub 2009 Mar 9. Cell Signal. 2009. PMID: 19275932 Free PMC article. - Tumor and stromal-based contributions to head and neck squamous cell carcinoma invasion.
Markwell SM, Weed SA. Markwell SM, et al. Cancers (Basel). 2015 Feb 27;7(1):382-406. doi: 10.3390/cancers7010382. Cancers (Basel). 2015. PMID: 25734659 Free PMC article. Review.
References
- Brasher B, Van Etten R. c-Abl has high intrinsic tyrosine kinase activity that is stimulated by mutation of the Src homology 3 domain and by autophosphorylation at two distinct regulatory tyrosines. J Biol Chem. 2000;275:631–637. - PubMed
- Buchdunger E, Zimmerman J, Mett H, Meyer T, Muller M, Druker B, et al. Inhibition of the Abl protein-tyrosine kinase in vitro and in vivo by a 2-phenylaminopyrimidine derivative. Cancer Res. 1996;56:100–104. - PubMed
- Das A, Sato M, Story M, Peyton M, Graves R, Redpath S, et al. Non-small cell lung cancers with kinase domain mutations in the epidermal growth factor receptor are sensitive to ionizing radiation. Cancer Res. 2006;66:9601–9608. - PubMed
- Dowell J, Minna J. Chasing mutations in the epidermal growth factor in lung cancer. N Eng J Med. 2005;352:830–832. - PubMed
- Feller SM, Ren R, Hanafusa H, Baltimore D. SH2 and SH3 domains as molecular adhesives: the interactions of Crk and Abl. Trends Biochem Sci. 1994;19:453–458. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous