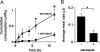Prandiology of Drosophila and the CAFE assay - PubMed (original) (raw)
Prandiology of Drosophila and the CAFE assay
William W Ja et al. Proc Natl Acad Sci U S A. 2007.
Abstract
Studies of feeding behavior in genetically tractable invertebrate model systems have been limited by the lack of proper methodology. We introduce the Capillary Feeder (CAFE), a method allowing precise, real-time measurement of ingestion by individual or grouped fruit flies on the scale of minutes to days. Using this technique, we conducted the first quantitative analysis of prandial behavior in Drosophila melanogaster. Our results allow the dissection of feeding into discrete bouts of ingestion, defining two separate parameters, meal volume and frequency, that can be uncoupled and thus are likely to be independently regulated. In addition, our long-term measurements show that flies can ingest as much as 1.7x their body mass over 24 h. Besides the study of appetite, the CAFE can be used to monitor oral drug delivery. As an illustration, we used the CAFE to test the effects of dietary supplementation with two compounds, paraquat and ethanol, on food ingestion and preference. Paraquat, a prooxidant widely used in stress tests, had a strong anorexigenic effect. In contrast, in a feeding preference assay, ethanol-laced food, but not ethanol by itself, acted as an attractant.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
The CAFE assay. (A) Schematic diagram. Liquid food, topped with an oil layer to minimize evaporation, is introduced via a glass capillary held in place by a pipette tip. The pierced bottom of the inner chamber provides humidity. (B) Fly feeding from the capillary. To facilitate visualization, a red dye has been added to the medium and can be seen in the proboscis and abdomen of the fly.
Fig. 2.
Prandial behavior analyzed in the CAFE. (A) Intake by three individually housed male flies fed 5% sucrose + 5% yeast extract measured in 10-min intervals. A vertical rise flanked by two intervals of no intake was defined as a meal. (B) Intake by individual flies fed 5% sucrose. (C) Meal volume and frequency can be decoupled by modulating nutrient conditions. On 5% sucrose, average meal size increases, whereas meal frequency is unchanged; 5% sucrose + 5% yeast extract: n = 10 flies, 52 meals; 5% sucrose: n = 4 flies, 18 meals. All values are given as averages ± SE. ∗, P ≤ 0.01, two-tailed t test.
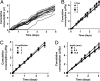
Fig. 3.
Measurement of long-term food consumption in the CAFE. (A) Cumulative ingestion by 17 individual male flies over 5 days. Average consumption = 1.5 ± 0.04 μl per day per fly. (B) The number of animals per chamber does not influence individual feeding rate. One, two, four, or eight flies were housed per CAFE. Average consumption was 2.0 ± 0.02, 2.1 ± 0.1, 2.3 ± 0.1, and 2.0 ± 0.1 μl per day per fly, respectively (_R_2 > 0.98 for each linear fit; ANOVA P = 0.24). (C) The number of capillaries per chamber does not affect food intake. One, two, or three capillaries were used per CAFE. Three flies were housed per chamber. Average consumption was 1.5 ± 0.03, 1.3 ± 0.04, and 1.3 ± 0.1 μl per day per fly, respectively (R_2 > 0.98; ANOVA P = 0.25). (D) Capillary depth has no effect on food ingestion. Four flies were used per CAFE, with the capillary tip placed 4 mm, 6.5 mm, or 16.5 mm below the top of the chamber, respectively (Fig. 1_A). Average consumption was 2.2 ± 0.2, 1.9 ± 0.1, and 1.8 ± 0.1 μl per day per fly, respectively (_R_2 > 0.99; ANOVA P = 0.26). In all experiments, 5% sucrose + 5% autolyzed yeast extract was served. All values are given as averages ± SE.
Fig. 4.
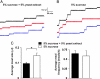
Dietary paraquat inhibits food intake. (A) Ingestion of a 5% sucrose solution with or without 20 mM paraquat over a 12-h period (n = 5 flies per condition. (B) Paraquat inhibits meal size. Consumption was recorded every 10 min during the first 6 h of the long-term experiment shown in A. All values are given as averages ± SE. ∗, P < 0.01, two-tailed t test.
Fig. 5.
Serving ethanol in the CAFE. (A) A dietary ethanol supplement has a modest, inhibitory effect on long-term food intake. Flies were fed 5% sucrose + 5% autolyzed yeast extract medium alone or supplemented with 1%, 5%, or 15% (vol/vol) ethanol. Average consumption was 1.7 ± 0.07, 1.7 ± 0.03, 1.4 ± 0.1, and 1.1 ± 0.01 μl per day per fly, respectively (_R_2 > 0.97 for each linear fit; ANOVA P = 0.018; n = 8 flies per condition). (B) In the absence of food medium, ingestion of either plain water or ethanol is remarkably low. For 24 h, flies were offered a choice between two capillaries, one containing pure water and the other containing one of three concentrations of ethanol: 1% (dotted line), 10% (dashed line), or 50% (solid line). Maximum ingestion was <0.07 μl per day per fly with 1% ethanol (n = 12 animals per condition). (C) Desiccation stimulates water consumption. Flies were deprived of food and water for 24 h in either a humidified or nonhumidified CAFE and then provided with plain water in regular humidified conditions. (D) Given a choice between food (5% sucrose + 5% autolyzed yeast extract) with and without a 15% ethanol supplement, flies showed a strong preference for the ethanol-laced regimen (n = 8 animals per condition). All values are given as averages ± SE. ∗, P < 0.05; ∗∗, P < 0.01, two-tailed t test.
Similar articles
- The CApillary FEeder Assay Measures Food Intake in Drosophila melanogaster.
Diegelmann S, Jansen A, Jois S, Kastenholz K, Velo Escarcena L, Strudthoff N, Scholz H. Diegelmann S, et al. J Vis Exp. 2017 Mar 17;(121):55024. doi: 10.3791/55024. J Vis Exp. 2017. PMID: 28362419 Free PMC article. - Taste preference for amino acids is dependent on internal nutritional state in Drosophila melanogaster.
Toshima N, Tanimura T. Toshima N, et al. J Exp Biol. 2012 Aug 15;215(Pt 16):2827-32. doi: 10.1242/jeb.069146. J Exp Biol. 2012. PMID: 22837455 - Simultaneous measurement of sleep and feeding in individual Drosophila.
Murphy KR, Park JH, Huber R, Ja WW. Murphy KR, et al. Nat Protoc. 2017 Nov;12(11):2355-2366. doi: 10.1038/nprot.2017.096. Epub 2017 Oct 12. Nat Protoc. 2017. PMID: 29022943 Free PMC article. - Does dietary restriction really increase longevity in Drosophila melanogaster?
Le Bourg E, Minois N. Le Bourg E, et al. Ageing Res Rev. 2005 Aug;4(3):409-21. doi: 10.1016/j.arr.2004.12.001. Ageing Res Rev. 2005. PMID: 16051527 Review. - Fruit fly behavior in response to chemosensory signals.
Herrero P. Herrero P. Peptides. 2012 Dec;38(2):228-37. doi: 10.1016/j.peptides.2012.09.019. Epub 2012 Sep 26. Peptides. 2012. PMID: 23022590 Review.
Cited by
- Dietary glucose regulates yeast consumption in adult Drosophila males.
Lebreton S, Witzgall P, Olsson M, Becher PG. Lebreton S, et al. Front Physiol. 2014 Dec 22;5:504. doi: 10.3389/fphys.2014.00504. eCollection 2014. Front Physiol. 2014. PMID: 25566097 Free PMC article. - Allatostatin A Signalling in Drosophila Regulates Feeding and Sleep and Is Modulated by PDF.
Chen J, Reiher W, Hermann-Luibl C, Sellami A, Cognigni P, Kondo S, Helfrich-Förster C, Veenstra JA, Wegener C. Chen J, et al. PLoS Genet. 2016 Sep 30;12(9):e1006346. doi: 10.1371/journal.pgen.1006346. eCollection 2016 Sep. PLoS Genet. 2016. PMID: 27689358 Free PMC article. - A neuroendocrine pathway modulating osmotic stress in Drosophila.
Zandawala M, Nguyen T, Balanyà Segura M, Johard HAD, Amcoff M, Wegener C, Paluzzi JP, Nässel DR. Zandawala M, et al. PLoS Genet. 2021 Mar 8;17(3):e1009425. doi: 10.1371/journal.pgen.1009425. eCollection 2021 Mar. PLoS Genet. 2021. PMID: 33684132 Free PMC article. - Sexual deprivation increases ethanol intake in Drosophila.
Shohat-Ophir G, Kaun KR, Azanchi R, Mohammed H, Heberlein U. Shohat-Ophir G, et al. Science. 2012 Mar 16;335(6074):1351-5. doi: 10.1126/science.1215932. Science. 2012. PMID: 22422983 Free PMC article. - A subset of brain neurons controls regurgitation in adult Drosophila melanogaster.
Chen YD, Ahmad S, Amin K, Dahanukar A. Chen YD, et al. J Exp Biol. 2019 Oct 1;222(Pt 19):jeb210724. doi: 10.1242/jeb.210724. J Exp Biol. 2019. PMID: 31511344 Free PMC article.
References
- Ewing LS, Ewing AW. Behaviour. 1987;101:243–252.
- Marella S, Fischler W, Kong P, Asgarian S, Rueckert E, Scott K. Neuron. 2006;49:285–295. - PubMed
- Klass MR. Mech Ageing Dev. 1983;22:279–286. - PubMed
- Dillin A, Hsu AL, Arantes-Oliveira N, Lehrer-Graiwer J, Hsin H, Fraser AG, Kamath RS, Ahringer J, Kenyon C. Science. 2002;298:2398–2401. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK070154/DK/NIDDK NIH HHS/United States
- AG24366/AG/NIA NIH HHS/United States
- R01 AG024366/AG/NIA NIH HHS/United States
- R01 DK070154/DK/NIDDK NIH HHS/United States
- R01 AG016630/AG/NIA NIH HHS/United States
- AG16630/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials