Inhibition of GLI-mediated transcription and tumor cell growth by small-molecule antagonists - PubMed (original) (raw)
Inhibition of GLI-mediated transcription and tumor cell growth by small-molecule antagonists
Matthias Lauth et al. Proc Natl Acad Sci U S A. 2007.
Abstract
The developmentally important Hedgehog (Hh) signaling pathway has recently been implicated in several forms of solid cancer. Current drug development programs focus on targeting the protooncogene Smoothened, a key transmembrane pathway member. These drug candidates, albeit promising, do not address the scenario in which pathway activation occurs downstream of Smoothened, as observed in cases of medulloblastoma, glioma, pericytoma, breast cancer, and prostate cancer. A cellular screen for small-molecule antagonists of GLI-mediated transcription, which constitutes the final step in the Hh pathway, revealed two molecules that are able to selectively inhibit GLI-mediated gene transactivation. We provide genetic evidence of downstream pathway blockade by these compounds and demonstrate the ineffectiveness of upstream antagonists such as cyclopamine in such situations. Mechanistically, both inhibitors act in the nucleus to block GLI function, and one of them interferes with GLI1 DNA binding in living cells. Importantly, the discovered compounds efficiently inhibited in vitro tumor cell proliferation in a GLI-dependent manner and successfully blocked cell growth in an in vivo xenograft model using human prostate cancer cells harboring downstream activation of the Hh pathway.
Conflict of interest statement
Conflict of interest statement: The inhibitors are the subject of a filed patent application.
Figures
Fig. 1.
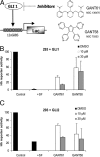
Inhibition of GLI-induced transcription in transfected HEK293 cells. (A) Schematic illustration of the compound screen to identify small-molecule GLI antagonists. GliBS, Gli binding site; Luc, firefly luciferase. The structures of two hits, GANT61 and GANT58, are given in the upper right. (B) GLI1 inhibition. (C) GLI2 (ΔN-GLI2) inhibition. SUFU (SF) was cotransfected as a positive control. To achieve equal transfection efficiencies in all wells, transfections were done on large plates and then split on smaller wells and treated. All values were normalized to total protein amount. Treatment time was 24 h, and control cells were treated with DMSO only. Shown is the mean of three independent experiments. Error bars indicate SD.
Fig. 2.
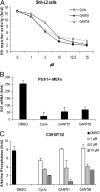
Inhibition of endogenous Hh signaling. (A) Dose–response curve of GANT61 and GANT58 in comparison to cyclopamine (Cyclo) in SAG-treated Shh-L2 cells. All three compounds are capable of inhibiting Hh signaling. Treatment time was 48 h, and normalization was against Renilla luciferase. Shown is the fold increase of Hh reporter activity compared with cells not treated with SAG. (B) Determination of Gli1 mRNA levels by quantitative PCR in Ptch1−/− MEFs (fold induction compared with wild-type MEFs). Confluent cells were treated with 10 μM compound for 2–3 days. Data were normalized to Gapdh expression. (C) Inhibition of SAG-induced alkaline phosphatase expression in C3H10T1/2 cells after treatment with compounds and SAG for 4 days. Values were normalized to total protein amount. Shown is the fold alkaline phosphatase induction compared with cells not treated with SAG. All experiments were repeated at least three times. Error bars depict SD.
Fig. 3.
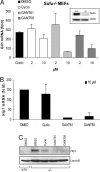
Downstream inhibition of the Hh pathway. (A and B) Quantitative RT-PCR for Gli1 mRNA (A) and Hip1 mRNA (B) in Sufu−/− MEFs treated with the indicated compounds for 3 days. Results were normalized against Gapdh mRNA levels. Shown is the fold increase in comparison to Sufu+/+ MEFs (n = at least three, error bars depict SD). (A Inset) Immunoblot verifying the absence of Sufu protein in Sufu−/− cells. (C) Hip1 Western blot of Sufu−/− MEFs treated with 10 μM compound.
Fig. 4.
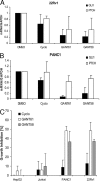
Inhibition of GLI-dependent human tumor cell growth. (A) Change of GAPDH-normalized GLI1 and PTCH expression in 22Rv1 and PANC1 cells on treatment with 5 μM compound for 48 h. (B) Inhibition of cell proliferation as measured by BrdU incorporation. Shown is the percentage of inhibition of BrdU incorporation in comparison to DMSO-treated samples (n = ≥4, error bars = indicate SD).
Fig. 5.
Human prostate cancer xenograft. (A) Change of tumor volume during treatment period. Time points of injections are given as arrows above the curve. (B) Macroscopic appearance of xenografts at the beginning of treatment (day 0) and at the end of treatment (day 18). No tumor could be seen in GANT61-treated animals (the bulge in the picture is part of the rib cage). (C) Quantification of PTCH mRNA by quantitative PCR in treated 22Rv1 tumors. Values were normalized against GAPDH. Shown is the mean of the analysis of two tumors for each treatment.
Fig. 6.
Inhibition of GLI1 DNA binding. (Upper Left) EMSA. Compounds were added to whole-cell lysates of GLI1-transfected HEK293 cells (in vitro). (Lower Left) Mean band intensities from two independent EMSA experiments. (Upper Right) EMSA. Compounds were added to Flag-GLI1-transfected HEK293 cells in culture (in vivo), and whole-cell lysates were prepared 24 h later. Lysate input was normalized to equal GLI1 loading. (Inset) Western blot (WB) using α-Flag. (Lower Right) Mean band intensities from two independent lysate preparations and EMSA experiments. GliBS, Gli binding site.
Similar articles
- Histone acetyltransferase PCAF is required for Hedgehog-Gli-dependent transcription and cancer cell proliferation.
Malatesta M, Steinhauer C, Mohammad F, Pandey DP, Squatrito M, Helin K. Malatesta M, et al. Cancer Res. 2013 Oct 15;73(20):6323-33. doi: 10.1158/0008-5472.CAN-12-4660. Epub 2013 Aug 13. Cancer Res. 2013. PMID: 23943798 - CDK7 inhibition suppresses aberrant hedgehog pathway and overcomes resistance to smoothened antagonists.
Liu F, Jiang W, Sui Y, Meng W, Hou L, Li T, Li M, Zhang L, Mo J, Wang J, Zhao Y, Zhang L, Ma J, Tang Y. Liu F, et al. Proc Natl Acad Sci U S A. 2019 Jun 25;116(26):12986-12995. doi: 10.1073/pnas.1815780116. Epub 2019 Jun 10. Proc Natl Acad Sci U S A. 2019. PMID: 31182587 Free PMC article. - Gli-1 expression is associated with lymph node metastasis and tumor progression in esophageal squamous cell carcinoma.
Mori Y, Okumura T, Tsunoda S, Sakai Y, Shimada Y. Mori Y, et al. Oncology. 2006;70(5):378-89. doi: 10.1159/000098111. Epub 2006 Dec 15. Oncology. 2006. PMID: 17179732 - Targeting GLI factors to inhibit the Hedgehog pathway.
Infante P, Alfonsi R, Botta B, Mori M, Di Marcotullio L. Infante P, et al. Trends Pharmacol Sci. 2015 Aug;36(8):547-58. doi: 10.1016/j.tips.2015.05.006. Epub 2015 Jun 10. Trends Pharmacol Sci. 2015. PMID: 26072120 Review. - Interference with HH-GLI signaling inhibits prostate cancer.
Stecca B, Mas C, Ruiz i Altaba A. Stecca B, et al. Trends Mol Med. 2005 May;11(5):199-203. doi: 10.1016/j.molmed.2005.03.004. Trends Mol Med. 2005. PMID: 15882606 Review.
Cited by
- Hedgehog signaling pathway in ovarian cancer.
Szkandera J, Kiesslich T, Haybaeck J, Gerger A, Pichler M. Szkandera J, et al. Int J Mol Sci. 2013 Jan 9;14(1):1179-96. doi: 10.3390/ijms14011179. Int J Mol Sci. 2013. PMID: 23303278 Free PMC article. Review. - Mitochondria-derived reactive oxygen species drive GANT61-induced mesothelioma cell apoptosis.
Lim CB, Prêle CM, Baltic S, Arthur PG, Creaney J, Watkins DN, Thompson PJ, Mutsaers SE. Lim CB, et al. Oncotarget. 2015 Jan 30;6(3):1519-30. doi: 10.18632/oncotarget.2729. Oncotarget. 2015. PMID: 25544756 Free PMC article. - GANT61 Reduces Hedgehog Molecule (GLI1) Expression and Promotes Apoptosis in Metastatic Oral Squamous Cell Carcinoma Cells.
Bacelar Sacramento de Araújo T, de Oliveira Siquara da Rocha L, Torres Andion Vidal M, Cerqueira Coelho PL, Galvão Dos Reis M, Solano de Freitas Souza B, Botelho Pereira Soares M, Almeida Pereira T, Della Coletta R, Pereira Bezerra D, Borges Dias R, Araújo Gurgel Rocha C. Bacelar Sacramento de Araújo T, et al. Int J Mol Sci. 2020 Aug 24;21(17):6076. doi: 10.3390/ijms21176076. Int J Mol Sci. 2020. PMID: 32846867 Free PMC article. - Modulation of Hedgehog Signaling for the Treatment of Basal Cell Carcinoma and the Development of Preclinical Models.
Dukes MW, Meade TJ. Dukes MW, et al. Biomedicines. 2022 Sep 23;10(10):2376. doi: 10.3390/biomedicines10102376. Biomedicines. 2022. PMID: 36289637 Free PMC article. Review. - Sonidegib, a novel smoothened inhibitor for the treatment of advanced basal cell carcinoma.
Doan HQ, Silapunt S, Migden MR. Doan HQ, et al. Onco Targets Ther. 2016 Sep 14;9:5671-5678. doi: 10.2147/OTT.S108171. eCollection 2016. Onco Targets Ther. 2016. PMID: 27695345 Free PMC article. Review.
References
- Beachy PA, Karhadkar SS, Berman DM. Nature. 2004;432:324–331. - PubMed
- Watkins DN, Berman DM, Burkholder SG, Wang B, Beachy PA, Baylin SB. Nature. 2003;422:313–317. - PubMed
- Berman DM, Karhadkar SS, Hallahan AR, Pritchard JI, Eberhart CG, Watkins DN, Chen JK, Cooper MK, Taipale J, Olson JM, et al. Science. 2002;297:1559–1561. - PubMed
- Berman DM, Karhadkar SS, Maitra A, Montes DO, Gerstenblith MR, Briggs K, Parker AR, Shimada Y, Eshleman JR, Watkins DN, et al. Nature. 2003;425:846–851. - PubMed
- Karhadkar SS, Bova GS, Abdallah N, Dhara S, Gardner D, Maitra A, Isaacs JT, Berman DM, Beachy PA. Nature. 2004;431:707–712. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical