Aging in adipocytes: potential impact of inherent, depot-specific mechanisms - PubMed (original) (raw)
Review
Aging in adipocytes: potential impact of inherent, depot-specific mechanisms
Mark J Cartwright et al. Exp Gerontol. 2007 Jun.
Abstract
Fat mass and tissue distribution change dramatically throughout life. Fat depot sizes reach a peak by middle or early old age, followed by a substantial decline, together with fat tissue dysfunction and redistribution in advanced old age. These changes are associated with health complications, including type 2 diabetes, atherosclerosis, dyslipidemia, thermal dysregulation, and skin ulcers, particularly in advanced old age. Fat tissue growth occurs through increases in size and number of fat cells. Fat cells turn over throughout the lifespan, with new fat cells developing from preadipocytes, which are of mesenchymal origin. The pool of preadipocytes comprises 15-50% of the cells in fat tissue. Since fat tissue turns over throughout life, characteristics of these cells very likely have a significant impact on fat tissue growth, plasticity, function, and distribution. The aims of this review are to highlight recent findings regarding changes in preadipocyte cell dynamics and function with aging, and to consider how inherent characteristics of these cells potentially contribute to age- and depot-dependent changes in fat tissue development and function.
Figures
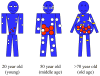
Figure 1. Diagram of age-associated changes in fat distribution
Fat mass reaches a peak by middle or early old age, followed by a substantial decline in advanced old age. Aging causes a loss of subcutaneous fat (peripherally first and then centrally), accumulation of visceral fat, and ectopic fat deposition (in muscle, liver, bone marrow, and elsewhere). White circles represent subcutaneous fat, red circles represent visceral fat, and yellow circles represent the appearance of fat in non adipose tissue.
Figure 2. Adipose tissue cell dynamics
Age-related changes in transcription factor function lead to reduced differentiation of preadipocytes.
Figure 3. Preadipocyte capacity for lipid accumulation declines with age
Differentiating preadipocytes isolated from young (3 month old), middle-aged (17 months), and old (24 months) Fischer 344 rat epididymal depots are shown.
Figure 4. Molecular mechanisms of age-related decreases in adipogenesis
Arrows and transcription factors in green indicate adipogenic pathways. Arrows and factors in red indicate antiadipogenic pathways. Age-related activation of cellular stress response pathways, coupled with increased preadipocyte cytokine generation, could trigger increased production of the antiadipogenic factors, C/EBPβ-LIP and CHOP. C/EBPβ-LIP and CHOP inhibit the production of the adipogenic transcription factors, PPARγ and C/EBPα, resulting in impaired adipogenesis and reduced expression of differentiation-dependent genes in fat cells. Reduced adipogenesis contributes to fat cell dysfunction, reduced fat depot size, and redistribution of fat to non-adipose tissues.
Similar articles
- Aging and regional differences in fat cell progenitors - a mini-review.
Sepe A, Tchkonia T, Thomou T, Zamboni M, Kirkland JL. Sepe A, et al. Gerontology. 2011;57(1):66-75. doi: 10.1159/000279755. Epub 2010 Jan 29. Gerontology. 2011. PMID: 20110661 Free PMC article. Review. - Increased TNFalpha and CCAAT/enhancer-binding protein homologous protein with aging predispose preadipocytes to resist adipogenesis.
Tchkonia T, Pirtskhalava T, Thomou T, Cartwright MJ, Wise B, Karagiannides I, Shpilman A, Lash TL, Becherer JD, Kirkland JL. Tchkonia T, et al. Am J Physiol Endocrinol Metab. 2007 Dec;293(6):E1810-9. doi: 10.1152/ajpendo.00295.2007. Epub 2007 Oct 2. Am J Physiol Endocrinol Metab. 2007. PMID: 17911345 - Aging, depot origin, and preadipocyte gene expression.
Cartwright MJ, Schlauch K, Lenburg ME, Tchkonia T, Pirtskhalava T, Cartwright A, Thomou T, Kirkland JL. Cartwright MJ, et al. J Gerontol A Biol Sci Med Sci. 2010 Mar;65(3):242-51. doi: 10.1093/gerona/glp213. Epub 2010 Jan 27. J Gerontol A Biol Sci Med Sci. 2010. PMID: 20106964 Free PMC article. - Preadipocyte function and aging: links between age-related changes in cell dynamics and altered fat tissue function.
Kirkland JL, Dobson DE. Kirkland JL, et al. J Am Geriatr Soc. 1997 Aug;45(8):959-67. doi: 10.1111/j.1532-5415.1997.tb02967.x. J Am Geriatr Soc. 1997. PMID: 9256849 Review. - Aging results in paradoxical susceptibility of fat cell progenitors to lipotoxicity.
Guo W, Pirtskhalava T, Tchkonia T, Xie W, Thomou T, Han J, Wang T, Wong S, Cartwright A, Hegardt FG, Corkey BE, Kirkland JL. Guo W, et al. Am J Physiol Endocrinol Metab. 2007 Apr;292(4):E1041-51. doi: 10.1152/ajpendo.00557.2006. Epub 2006 Dec 5. Am J Physiol Endocrinol Metab. 2007. PMID: 17148751
Cited by
- In utero programming of later adiposity: the role of fetal growth restriction.
Sarr O, Yang K, Regnault TR. Sarr O, et al. J Pregnancy. 2012;2012:134758. doi: 10.1155/2012/134758. Epub 2012 Nov 1. J Pregnancy. 2012. PMID: 23251802 Free PMC article. Review. - p53 Functions in Adipose Tissue Metabolism and Homeostasis.
Krstic J, Reinisch I, Schupp M, Schulz TJ, Prokesch A. Krstic J, et al. Int J Mol Sci. 2018 Sep 4;19(9):2622. doi: 10.3390/ijms19092622. Int J Mol Sci. 2018. PMID: 30181511 Free PMC article. Review. - Proliferation of nutrition sensing preadipocytes upon short term HFD feeding.
Kulenkampff E, Wolfrum C. Kulenkampff E, et al. Adipocyte. 2019 Dec;8(1):16-25. doi: 10.1080/21623945.2018.1521229. Epub 2018 Sep 29. Adipocyte. 2019. PMID: 30269635 Free PMC article. - Interventions for Body Composition and Upper and Lower Extremity Muscle Strength in Older Adults in Rural Taiwan: A Horizontal Case Study.
Chen CA, Lai MC, Huang H, Wu CE. Chen CA, et al. Int J Environ Res Public Health. 2022 Jun 27;19(13):7869. doi: 10.3390/ijerph19137869. Int J Environ Res Public Health. 2022. PMID: 35805529 Free PMC article. Clinical Trial. - Naturally occurring compensated insulin resistance selectively alters glucose transporters in visceral and subcutaneous adipose tissues without change in AS160 activation.
Waller AP, Kohler K, Burns TA, Mudge MC, Belknap JK, Lacombe VA. Waller AP, et al. Biochim Biophys Acta. 2011 Sep;1812(9):1098-103. doi: 10.1016/j.bbadis.2011.02.007. Epub 2011 Feb 23. Biochim Biophys Acta. 2011. PMID: 21352908 Free PMC article.
References
- Adams M, Montague CT, Prins JB, Holder JC, Smith SA, Sanders L, Digby JE, Sewter CP, Lazar MA, Chatterjee VK, O’Rahilly S. Activators of peroxisome proliferator-activated receptor gamma have depot-specific effects on human preadipocyte differentiation. J Clin Invest. 1997;100:3149–3153. - PMC - PubMed
- Bryhni B, Jenssen TG, Olafsen K, Bendikssen A. Oxidative and nonoxidative glucose disposal in elderly vs younger men with similar and smaller body mass indices and waist circumferences. Metabolism. 2005;54:748–755. - PubMed
- Burgess-Beusse B, Timchenko NA, Darlington GJ. CCAAT/enhancer binding protein alpha (C/EBPalpha) is an important mediator of mouse C/EBPbeta protein isoform production. Hepatology. 1999;29:597–601. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AG13925/AG/NIA NIH HHS/United States
- R01 DK056891/DK/NIDDK NIH HHS/United States
- R01 AG023960/AG/NIA NIH HHS/United States
- R37 AG013925/AG/NIA NIH HHS/United States
- AG23960/AG/NIA NIH HHS/United States
- R01 AG013925-09/AG/NIA NIH HHS/United States
- DK56891/DK/NIDDK NIH HHS/United States
- R01 AG023960-04S1/AG/NIA NIH HHS/United States
- R01 DK056891-05/DK/NIDDK NIH HHS/United States
- R01 AG013925/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical