Phosphatidylinositol-4,5-bisphosphate regulates NMDA receptor activity through alpha-actinin - PubMed (original) (raw)
Comparative Study
Phosphatidylinositol-4,5-bisphosphate regulates NMDA receptor activity through alpha-actinin
Ioannis E Michailidis et al. J Neurosci. 2007.
Abstract
Phosphatidylinositol-4,5-bisphosphate (PIP2) has been shown to regulate many ion channels, transporters, and other signaling proteins, but it is not known whether it also regulates neurotransmitter-gated channels. The NMDA receptors (NMDARs) are gated by glutamate and serve as a critical control point in synaptic function. Here we demonstrate that PIP2 supports NMDAR activity. In Xenopus oocytes, overexpression of phospholipase Cgamma (PLCgamma) or preincubation with 10 microm wortmannin markedly reduced NMDA currents. Stimulation of the epidermal growth factor receptor (EGFR) promoted the formation of an immunocomplex between PLCgamma and NMDAR subunits. Stimulation of EGFR or the PLCbeta-coupled M1 acetylcholine receptor produced a robust transient inhibition of NMDA currents. Wortmannin application blocked the recovery of NMDA currents from the inhibition. Using mutagenesis, we identified the structural elements on NMDAR intracellular tails that transduce the receptor-mediated inhibition, which pinpoint to the binding site for the cytoskeletal protein alpha-actinin. Mutation of the PIP2-binding residues of alpha-actinin dramatically reduced NMDA currents and occluded the effect of EGF. Interestingly, EGF or wortmannin affected the interaction between NMDAR subunits and alpha-actinin, suggesting that this protein mediates the effect of PIP2 on NMDARs. In mature hippocampal neurons, expression of the mutant alpha-actinin reduced NMDA currents and accelerated inactivation. We propose a model in which alpha-actinin supports NMDAR activity via tethering their intracellular tails to plasma membrane PIP2. Thus, our results extend the influence of PIP2 to the NMDA ionotropic glutamate receptors and introduce a novel mechanism of "indirect" regulation of transmembrane protein activity by PIP2.
Figures
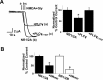
Figure 1.
PLCγ inhibits NMDA currents in Xenopus laevis oocytes. A, Left, Representative traces of whole-cell NR1/2A currents recorded with the two-electrode voltage-clamp technique at −80 mV (in this and subsequent figures). NR1/2A currents were evoked by NMDA (100 μ
m
) and glycine (Gly; 10 μ
m
) applied as indicated by arrows, in control cells (b), cells overexpressing PLCγ (a), or cells expressing the inactive mutant PLCγY783F (c), as described in Results. Right, Bars representing mean ± SE percentage steady-state current after agonist application, normalized to the average of the control group. The means for the PLCγ group were significantly different (*p < 0.01, t test). B, Bars obtained similarly as in A but for NR1/2A or NR1/2B currents from control oocytes or oocytes preincubated for 1 h with 10 μ
m
wortmannin (Wtmn). The means were significantly different in all sets of experiments (p < 0.001, t test).
Figure 2.
Stimulation of hormonal receptors that activate PLC transiently inhibits NMDA currents. A, Representative traces of the effect to the NR1/2A current of 100 ng/ml EGF or 5 μ
m
ACh applied as shown. Bars represent mean ± SE percentage current of current before (control current, taken as always 100% before EGF or ACh application in this and subsequent figures when comparison with inhibited states is made) or of maximally inhibited current after EGF or ACh application. Both means representing inhibited current were significantly different from control (*p < 0.001, t test). B, Xenopus oocytes expressing NR1/2A subunits and EGFR were stimulated with EGF (100 ng/ml) as indicated, and membrane preparations were obtained as described in Materials and Methods. Top, Western immunoblot (WB) of a sample of the membrane preparations with an antibody against NR1. The rest of the same membrane preparations were subjected to immunoprecipitation (IP) using an antibody against PLCγ (see Materials and Methods). Bottom, NR1 Western blotting of oocyte membrane immunoprecipitates pulled down with PLCγ antibodies. Blots are representative of two independent experiments with similar results.
Figure 3.
Wortmannin blocks the recovery of NMDA currents in response to PLC-coupled receptor stimulation. A, B, Left traces, Effect to the NR1/2C current of 100 ng/ml EGF or 5 μ
m
ACh applied as indicated. Right traces, In addition to EGF or ACh, wortmannin (Wtmn; 20–40 μ
m
) was perfused to the oocyte (different oocytes from the ones used in experiments without wortmannin) as indicated. Bars represent mean ± SE percentage current of current before (control current) or of maximally inhibited current after EGF or ACh application (I) or of recovered current (R) from cells with or without wortmannin (I, R + wortmannin). *p < 0.001, the mean maximally recovered currents in cells perfused with wortmannin were significantly different than control cells (for both EGF and Ach; t test). The arrows indicate where the I and R measurements were made. Gly, Glycine.
Figure 4.
Intracellular Ca2+ store depletion does not prevent NMDA current inhibition in response to PLC-coupled receptor stimulation. A, B, Traces represent NMDA currents recorded from oocytes expressing NR1/2A (A) or NR1/2B (B) subunits on which EGF or ACh were administered as indicated. Thapsigargin (1–5 μ
m
) was used to pretreat the oocytes for 30–60 min before experiments. C, Bars represent mean ± SE percentage current of current before (control current) or of maximally inhibited current after EGF (100 ng/ml) or ACh (5 μ
m
) application. All sets of means were significantly different (*p < 0.001, t test). Gly, Glycine.
Figure 5.
The C0 domain of the NR1 subunit is required for the inhibition of NR1/2A currents. A, The C terminus of NR1 (NR1–1b). Shown in bold are the α-actinin-binding residues within C0. B, Traces represent recordings of NMDA currents from oocytes expressing the NR1 deletion mutant subunits indicated (see Results) with wild-type NR2A subunit, in which EGF (1 ng/ml) or ACh (5 μ
m
) were applied as indicated. In NR1838 and NR1ΔC0, the C terminus of NR1 or only its C0 domain have been deleted, respectively. Bars represent mean ± SE percentage current of current before (control current) or of current after EGF or ACh application. The means were not significantly different (t test). Gly, Glycine; TM, transmembrane domain.
Figure 6.
α-Actinin-binding sites within the C0 domain of NR1 and the distal α-actinin-binding region of the C terminus of NR2B are required for inhibition of NR1/2B currents. A–C, Traces represent recordings of NMDA currents from thapsigargin-treated oocytes expressing wild-type NR1 or NR1 deletion mutants, together with wild-type NR2B or an NR2B mutant as indicated. EGF (100 ng/ml) or ACh (5 μ
m
) were applied as indicated. The constructs have been named after the last residue expressed in the mutant subunit (see Results). D, Bars represent mean ± SE percentage current of current before (control current) or of steady-state current after EGF or ACh application. The means were significantly different only for NR1 or NR2B subunit combinations that contained an α-actinin-binding site (p < 0.01, t test). D, Schematic of NR1 and NR2B subunits with sites of mutants used. α-Actinin binds residues 858–863 of NR1 (see also Fig. 5) and the distal C terminus of NR2B. Gly, Glycine; TM, transmembrane domain.
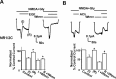
Figure 7.
Mutation of the PIP2-binding residues of α-actinin affects NMDA currents in oocytes. Top left, Bars representing mean ± SE percentage steady-state normalized NMDA current recorded from oocytes expressing NR1/2A together with endogenous α-actinin (control), overexpressed wild-type α-actinin 2, single-point mutant α-actinin 2 K184Q, or double-point mutant α-actinin 2 R172Q–K184Q. The means were significantly different from control (p < 0.001 for all comparisons, t tests). Top right, Representative NMDA current trace of a control oocyte expressing NR1/2A in response to EGF (1 ng/ml) applied as indicated. Bottom left, Representative NMDA current trace of an oocyte expressing NR1/2A and single-point mutant α-actinin 2 K184Q in response to 1 ng/ml EGF. Bottom right, Bars represent mean ± SE percentage current of current before (control current) or of steady-state current after EGF application from oocytes expressing NR1/2A, and NR1/2A together with wild-type α-actinin 2 or single-point mutant α-actinin 2 K184Q. *p < 0.001, statistically significant difference between the NR1/2A group and the K184Q group (t test).
Figure 8.
Disruption of the α-actinin–PIP2 interaction affects NMDA currents in hippocampal neurons. A, Overlaid normalized representative NMDA–glycine-evoked current traces from neurons clamped at −60 mV expressing α-actinin–EGFP (red) or the α-actinin–EGFP R172Q–K184Q mutant (blue). For α-actinin–EGFP and α-actinin–EGFP R172Q–K184Q expressing neurons compared are mean ± SE. B, Percentage normalized peak NMDAR/K+ channel current ratios obtained as described in Materials and Methods. C, Percentage of the peak NMDA current remaining 1 s after peak. D, Time constants (tau) of inactivation of agonist-evoked NMDAR currents in neurons. All pairs of means were significantly different (*p < 0.001, t test). Gly, Glycine; α-act, α-actinin.
Comment in
- Phosphatidylinositol-4,5-bisphosphate and alpha-actinin: two-component hinge for the NMDA receptor.
Gao L. Gao L. J Neurosci. 2007 Sep 26;27(39):10321-2. doi: 10.1523/JNEUROSCI.2784-07.2007. J Neurosci. 2007. PMID: 17898203 Free PMC article. No abstract available.
Similar articles
- Phosphatidylinositol [correction] 4,5-bisphosphate signals underlie receptor-specific Gq/11-mediated modulation of N-type Ca2+ channels.
Gamper N, Reznikov V, Yamada Y, Yang J, Shapiro MS. Gamper N, et al. J Neurosci. 2004 Dec 1;24(48):10980-92. doi: 10.1523/JNEUROSCI.3869-04.2004. J Neurosci. 2004. PMID: 15574748 Free PMC article. - Phosphatidylinositol-4,5-bisphosphate and alpha-actinin: two-component hinge for the NMDA receptor.
Gao L. Gao L. J Neurosci. 2007 Sep 26;27(39):10321-2. doi: 10.1523/JNEUROSCI.2784-07.2007. J Neurosci. 2007. PMID: 17898203 Free PMC article. No abstract available. - Phosphatidylinositol 4,5-bisphosphate depletion fails to affect neurosteroid modulation of GABAA receptor function.
Mennerick S, Taylor AA, Zorumski CF. Mennerick S, et al. Psychopharmacology (Berl). 2014 Sep;231(17):3493-501. doi: 10.1007/s00213-014-3486-5. Epub 2014 Feb 20. Psychopharmacology (Berl). 2014. PMID: 24553581 Free PMC article. - Role of c-Src in cellular events associated with colony-stimulating factor-1-induced spreading in osteoclasts.
Insogna K, Tanaka S, Neff L, Horne W, Levy J, Baron R. Insogna K, et al. Mol Reprod Dev. 1997 Jan;46(1):104-8. doi: 10.1002/(SICI)1098-2795(199701)46:1<104::AID-MRD16>3.0.CO;2-2. Mol Reprod Dev. 1997. PMID: 8981371 Review. - Functional interactions of ion channels with the actin cytoskeleton: does coupling to dynamic actin regulate NMDA receptors?
Shaw JE, Koleske AJ. Shaw JE, et al. J Physiol. 2021 Jan;599(2):431-441. doi: 10.1113/JP278702. Epub 2020 Mar 12. J Physiol. 2021. PMID: 32034761 Free PMC article. Review.
Cited by
- Subtype-specific regulation of P2X3 and P2X2/3 receptors by phosphoinositides in peripheral nociceptors.
Mo G, Bernier LP, Zhao Q, Chabot-Doré AJ, Ase AR, Logothetis D, Cao CQ, Séguéla P. Mo G, et al. Mol Pain. 2009 Aug 11;5:47. doi: 10.1186/1744-8069-5-47. Mol Pain. 2009. PMID: 19671169 Free PMC article. - Phosphatidylinositol (4,5)-bisphosphate regulation of N-methyl-D-aspartate receptor channels in cortical neurons.
Mandal M, Yan Z. Mandal M, et al. Mol Pharmacol. 2009 Dec;76(6):1349-59. doi: 10.1124/mol.109.058701. Epub 2009 Sep 21. Mol Pharmacol. 2009. PMID: 19770351 Free PMC article. - Differentially expressed genes in Hirudo medicinalis ganglia after acetyl-L-carnitine treatment.
Federighi G, Macchi M, Bernardi R, Scuri R, Brunelli M, Durante M, Traina G. Federighi G, et al. PLoS One. 2013;8(1):e53605. doi: 10.1371/journal.pone.0053605. Epub 2013 Jan 4. PLoS One. 2013. PMID: 23308261 Free PMC article. - Cross-Talk between P2X and NMDA Receptors.
Rodriguez L, Yi C, Chu C, Duriez Q, Watanabe S, Ryu M, Reyes B, Asatryan L, Boué-Grabot E, Davies D. Rodriguez L, et al. Int J Mol Sci. 2020 Sep 29;21(19):7187. doi: 10.3390/ijms21197187. Int J Mol Sci. 2020. PMID: 33003406 Free PMC article. - Lipid agonism: The PIP2 paradigm of ligand-gated ion channels.
Hansen SB. Hansen SB. Biochim Biophys Acta. 2015 May;1851(5):620-8. doi: 10.1016/j.bbalip.2015.01.011. Epub 2015 Jan 26. Biochim Biophys Acta. 2015. PMID: 25633344 Free PMC article. Review.
References
- Balla T. Phosphatidylinositol 4-kinases. Biochim Biophys Acta. 1998;1436:69–85. - PubMed
- Buonanno A, Fischbach GD. Neuregulin and ErbB receptor signaling pathways in the nervous system. Curr Opin Neurobiol. 2001;11:287–296. - PubMed
- Cochet C, Filhol O, Payrastre B, Hunter T, Gill GN. Interaction between the epidermal growth factor receptor and phosphoinositide kinases. J Biol Chem. 1991;266:637–644. - PubMed
- Ding WG, Toyoda F, Matsuura H. Regulation of cardiac IKs potassium current by membrane phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 2004;279:50726–50734. - PubMed
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous