A developmentally regulated chaperone complex for the endoplasmic reticulum of male haploid germ cells - PubMed (original) (raw)
A developmentally regulated chaperone complex for the endoplasmic reticulum of male haploid germ cells
Marcel van Lith et al. Mol Biol Cell. 2007 Aug.
Abstract
Glycoprotein folding is mediated by lectin-like chaperones and protein disulfide isomerases (PDIs) in the endoplasmic reticulum. Calnexin and the PDI homologue ERp57 work together to help fold nascent polypeptides with glycans located toward the N-terminus of a protein, whereas PDI and BiP may engage proteins that lack glycans or have sugars toward the C-terminus. In this study, we show that the PDI homologue PDILT is expressed exclusively in postmeiotic male germ cells, in contrast to the ubiquitous expression of many other PDI family members in the testis. PDILT is induced during puberty and represents the first example of a PDI family member under developmental control. We find that PDILT is not active as an oxido-reductase, but interacts with the model peptide Delta-somatostatin and nonnative bovine pancreatic trypsin inhibitor in vitro, indicative of chaperone activity. In vivo, PDILT forms a tissue-specific chaperone complex with the calnexin homologue calmegin. The identification of a redox-inactive chaperone partnership defines a new system of testis-specific protein folding with implications for male fertility.
Figures
Figure 1.
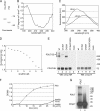
PDILT has chaperone-like properties. (A) Purified human His-tagged PDILT and purified rat PDI were analyzed by SDS-PAGE and Coomassie staining. (B) Far-UV circular dichroism spectrum of purified recombinant human PDILT. (C) Fluorescence spectra of purified human His-tagged PDILT under native (−Gnd) and denaturing conditions (+Gnd). (D) Guanidine hydrochloride denaturing curve of purified human His-tagged PDILT. Shown is the ratio of the average fluorescence intensity at 332–336 nm (peak of native protein) to that at 320–400 nm. (E) HeLa cells were mock-transfected or transfected with myc-tagged wild-type PDILT or the PDILT cysteine mutants as indicated. Lysates of these transfectants were analyzed by nonreducing (lanes 1–5) and reducing (lanes 6–10) SDS-PAGE. Monomeric PDILT (PDILT-SH) and PDILT in disulfide-linked complexes (PDILT-SS-*) are indicated. Molecular weights in kDa are indicated on the right-hand side. (F) Human insulin, 0.17 mM, was incubated with DTT only (insulin) or DTT with 1 μM enzyme (PDI or PDILT) in PBS. Insulin without DTT (insulin-DTT) was indistinguishable from PBS. The OD600 was monitored over 30 min to follow precipitation of the insulin B chain. (G) Extracts of E. coli expressing ERp27 were incubated with 125I-labeled Δ-somatostatin with (lane 1) or without (lane 2) 2 μg recombinant PDILT. After cross-linking with disuccinimidyl glutarate, the samples were analyzed by SDS-PAGE.
Figure 2.
Specificity of the PDILT antiserum. (A) Lysates from HeLa cells transfected with PDILT-myc or an irrelevant myc construct were analyzed by Western blotting with a rabbit antiserum raised against purified recombinant His-tagged human PDILT. (B) Equal amounts (in mg of tissue) of murine tissue lysates were analyzed by Western blotting with a rabbit antiserum raised against purified recombinant His-tagged human PDILT (top), anti-PDI (middle), and anti-calnexin (bottom).
Figure 3.
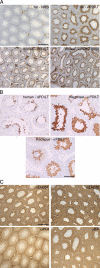
Expression of PDILT is germ cell specific. (A) Sections from rat testis counterstained with hematoxylin (blue) were immunostained with normal rabbit serum (NRS) or an anti-PDILT serum raised against recombinant PDILT at a dilution of 1:8000 (top panels). The PDILT staining pattern (brown) is similar for mouse testis (bottom left) or when using a serum raised against an internal PDILT peptide at 1:1000 (bottom right). Scale bar, 200 μm. (B) Testis sections from human, macaque, and marmoset were immunostained for PDILT. Although a single population of PDILT-positive germ cells occupied the full circumference of the tubule in macaque, the staining on human and marmoset was more heterogenous consistent with the existence of tubules containing more than one stage of spermatogenesis in a single cross section. Scale bar, 100 μm. (C) Rat testis sections were immunostained for ERp57, ERp72, PDI, and BiP (brown). Expression of these proteins was observed throughout the seminiferous epithelium. Scale bar, 200 μm.
Figure 4.
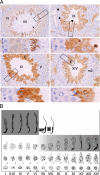
Germ cell expression of PDILT from stage VII. (A) Rat testis sections immunostained with anti-PDILT from Figure 3A were imaged at higher magnification. Four representative sections from stages VII, IX, XI, and XIV of the rat spermatogenic cycle are shown. A higher detailed section, indicated by a box, is shown below each stage. Round spermatids (R), elongate spermatids (E), and immunonegative pachytene spermatocytes (P) and germ cells undergoing meiotic division (m2) are indicated. At stage VII mature elongate spermatids ready for release are indicated by arrows. At stage IX immunopositive staining was particularly intense in small areas (arrowheads) that are likely to be the residual cytoplasmic remnants from spermatozoa released at stage VII. (B) A staging diagram showing the germ cell associations characteristic of the spermatogenic cycle (Russell et al., 1990) summarizes the expression levels of PDILT (gray shading) in the adult rat.
Figure 5.
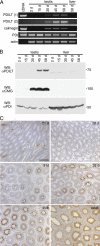
Expression of PDILT commences during the first wave of spermatogenesis. (A) Total RNA extracted from testis of 2-, 15-, 30-, 45-, and 58-d-old rats was used for RT-PCR using primer sets specific for PDILT, calmegin, PDI, and actin. As a positive control, cDNA was used for PDILT, calmegin, and PDI. PDILT primer set 1 was designed on 2 internal exons, whereas PDILT primer set 2 was designed on the first and last exons, to exclude expression of alternatively spliced variants during development. (B) Testis and liver from 2-, 15-, 30-, 45-, and 58-d-old rats were lysed and analyzed by Western blotting for PDILT, calmegin, and PDI. (C) Testis sections from 21-, 28-, 31-, 38-, 45-d-old and adult rats were counterstained with hematoxylin (blue) and immunostained for PDILT (brown).
Figure 6.
PDILT self-associates. Wild-type (wt) or cysteine mutant (C135A/C420A, denoted CA) HA- or myc-tagged PDILT was expressed in HeLa cells as indicated. Lysates were directly analyzed (lanes 1–4) or subjected to immunoprecipitation with anti-myc (lanes 5–8) or anti-HA (lanes 9–12) before SDS-PAGE and Western blotting with anti-HA (bottom panel) and anti-myc (top panel). The asterisk indicates the bands for the antibodies used for the immunoprecipitations.
Figure 7.
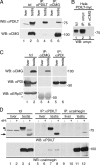
PDILT interacts with calmegin. (A) Lysates from mouse liver and testis were either directly analyzed (lanes l and 2) or subjected to immunoprecipitation with anti-PDILT (lanes 3 and 4) or anti-calmegin (lanes 5 and 6) before analysis by SDS-PAGE and Western blotting with anti-PDILT (top panel) and anti-calmegin (bottom panel). (B) Lysates from HeLa cells transfected with PDILT-myc were directly analyzed (lane 1) or subjected to immunoprecipitation with anti-myc (lane 2) and anti-calmegin (lane 3). All samples were analyzed by SDS-PAGE and Western blotting with anti-myc. (C) Lysates from rat liver and testis were either directly analyzed (lanes 1 and 2) or subjected to immunoprecipitation with anti-calmegin (lanes 3 and 4) or anti-PDI (lanes 5 and 6) before analysis by SDS-PAGE and Western blotting with anti-calmegin, anti-PDI (mouse monoclonal), and anti-ERp57. The asterisk indicates cross-reactive antibodies used for the immunoprecipitation in lanes 3 and 4. (D) Lysates from mouse liver and testis were treated with endoH and either directly analyzed (lanes 1–4) or subjected to immunoprecipitation with anti-PDILT (lanes 5–8) or anti-calmegin (lanes 9–12) before analysis by SDS-PAGE and Western blotting with anti-PDILT (top panel) and anti-calmegin (bottom panel). The arrowhead indicates glycosylated PDILT, and the arrow indicates deglycosylated PDILT. The asterisk indicates a background band occasionally observed.
Similar articles
- PDILT, a divergent testis-specific protein disulfide isomerase with a non-classical SXXC motif that engages in disulfide-dependent interactions in the endoplasmic reticulum.
van Lith M, Hartigan N, Hatch J, Benham AM. van Lith M, et al. J Biol Chem. 2005 Jan 14;280(2):1376-83. doi: 10.1074/jbc.M408651200. Epub 2004 Oct 8. J Biol Chem. 2005. PMID: 15475357 - Enhanced catalysis of ribonuclease B folding by the interaction of calnexin or calreticulin with ERp57.
Zapun A, Darby NJ, Tessier DC, Michalak M, Bergeron JJ, Thomas DY. Zapun A, et al. J Biol Chem. 1998 Mar 13;273(11):6009-12. doi: 10.1074/jbc.273.11.6009. J Biol Chem. 1998. PMID: 9497314 - Protein disulfide isomerase homolog PDILT is required for quality control of sperm membrane protein ADAM3 and male fertility [corrected].
Tokuhiro K, Ikawa M, Benham AM, Okabe M. Tokuhiro K, et al. Proc Natl Acad Sci U S A. 2012 Mar 6;109(10):3850-5. doi: 10.1073/pnas.1117963109. Epub 2012 Feb 22. Proc Natl Acad Sci U S A. 2012. PMID: 22357757 Free PMC article. - Protein disulfide-isomerase, a folding catalyst and a redox-regulated chaperone.
Wang L, Wang X, Wang CC. Wang L, et al. Free Radic Biol Med. 2015 Jun;83:305-13. doi: 10.1016/j.freeradbiomed.2015.02.007. Epub 2015 Feb 17. Free Radic Biol Med. 2015. PMID: 25697778 Review. - Protein disulfide isomerases: Redox connections in and out of the endoplasmic reticulum.
Soares Moretti AI, Martins Laurindo FR. Soares Moretti AI, et al. Arch Biochem Biophys. 2017 Mar 1;617:106-119. doi: 10.1016/j.abb.2016.11.007. Epub 2016 Nov 24. Arch Biochem Biophys. 2017. PMID: 27889386 Review.
Cited by
- Structural insight into the dimerization of human protein disulfide isomerase.
Bastos-Aristizabal S, Kozlov G, Gehring K. Bastos-Aristizabal S, et al. Protein Sci. 2014 May;23(5):618-26. doi: 10.1002/pro.2444. Epub 2014 Mar 11. Protein Sci. 2014. PMID: 24549644 Free PMC article. - Structure of the substrate-binding b' domain of the Protein Disulfide Isomerase-Like protein of the Testis.
Bastos-Aristizabal S, Kozlov G, Gehring K. Bastos-Aristizabal S, et al. Sci Rep. 2014 Mar 25;4:4464. doi: 10.1038/srep04464. Sci Rep. 2014. PMID: 24662985 Free PMC article. - Chronic exercise training attenuates prostate cancer-induced molecular remodelling in the testis.
Matos B, Patrício D, Henriques MC, Freitas MJ, Vitorino R, Duarte IF, Howl J, Oliveira PA, Seixas F, Duarte JA, Ferreira R, Fardilha M. Matos B, et al. Cell Oncol (Dordr). 2021 Apr;44(2):311-327. doi: 10.1007/s13402-020-00567-9. Epub 2020 Oct 19. Cell Oncol (Dordr). 2021. PMID: 33074478 - TMX4-driven LINC complex disassembly and asymmetric autophagy of the nuclear envelope upon acute ER stress.
Kucińska MK, Fedry J, Galli C, Morone D, Raimondi A, Soldà T, Förster F, Molinari M. Kucińska MK, et al. Nat Commun. 2023 Jun 13;14(1):3497. doi: 10.1038/s41467-023-39172-3. Nat Commun. 2023. PMID: 37311770 Free PMC article. - The control of male fertility by spermatid-specific factors: searching for contraceptive targets from spermatozoon's head to tail.
Chen SR, Batool A, Wang YQ, Hao XX, Chang CS, Cheng CY, Liu YX. Chen SR, et al. Cell Death Dis. 2016 Nov 10;7(11):e2472. doi: 10.1038/cddis.2016.344. Cell Death Dis. 2016. PMID: 27831554 Free PMC article. Review.
References
- Adham I. M., Nayernia K., Burkhardt-Gottges E., Topaloglu O., Dixkens C., Holstein A. F., Engel W. Teratozoospermia in mice lacking the transition protein 2 (Tnp2) Mol. Hum. Reprod. 2001;7:513–520. - PubMed
- Alanen H. I., Williamson R. A., Howard M. J., Hatahet F. S., Salo K. E., Kauppila A., Kellokumpu S., Ruddock L. W. ERp27, a new non-catalytic endoplasmic reticulum-located human protein disulfide isomerase family member, interacts with ERp57. J. Biol. Chem. 2006;281:33727–33738. - PubMed
- Brohmann H., Pinnecke S., Hoyer-Fender S. Identification and characterization of new cDNAs encoding outer dense fiber proteins of rat sperm. J. Biol. Chem. 1997;272:10327–10332. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous