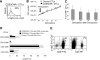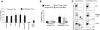Epstein Barr virus specific cytotoxic T lymphocytes expressing the anti-CD30zeta artificial chimeric T-cell receptor for immunotherapy of Hodgkin disease - PubMed (original) (raw)
Epstein Barr virus specific cytotoxic T lymphocytes expressing the anti-CD30zeta artificial chimeric T-cell receptor for immunotherapy of Hodgkin disease
Barbara Savoldo et al. Blood. 2007.
Abstract
Adoptive transfer of Epstein Barr virus (EBV)-specific cytotoxic T-lymphocytes (EBV-CTLs) has shown that these cells persist in patients with EBV(+) Hodgkin lymphoma (HD) to produce complete tumor responses. Treatment failure, however, occurs if a subpopulation of malignant cells in the tumor lacks or loses expression of EBV antigens. We have therefore determined whether we could prepare EBV-CTLs that retained the antitumor activity conferred by their native receptor while expressing a chimeric antigen receptor (CAR) specific for CD30, a molecule highly and consistently expressed on malignant Hodgkin Reed-Sternberg cells. We made a CD30CAR and were able to express it on 26% (+/- 11%) and 22% (+/- 5%) of EBV-CTLs generated from healthy donors and HD patients, respectively. These CD30CAR(+) CTLs killed both autologous EBV(+) cells through their native receptor and EBV(-)/CD30(+) targets through their major histocompatibility complex (MHC)-unrestricted CAR. A subpopulation of activated T cells also express CD30, but the CD30CAR(+) CTLs did not impair cellular immune responses, probably because normal T cells express lower levels of the target antigen. In a xenograft model, CD30CAR(+) EBV-CTLs could be costimulated by EBV-infected cells and produce antitumor effects even against EBV(-)/CD30(+) tumors. EBV-CTLs expressing both a native and a chimeric antigen receptor may therefore have added value for treatment of HD.
Figures
Figure 1
Growth kinetics, immunophenotype, and functionality of EBV-CTL lines are retained after transduction with CD30CAR. EBV-CTL lines were expanded from PBMCs obtained from 8 healthy EBV-seropositive donors by weekly stimulation with irradiated autologous LCLs and biweekly feeding with rhIL-2. EBV-CTLs were transduced with the CD30CAR or an irrelevant CAR (control CTLs) after the third stimulation. (A) CD30CAR expression was evaluated by flow cytometry using a goat anti–human IgG (H + L) Ab (—) or the isotype control (- - -). (B) The growth of EBV-CTLs was transduced with the CD30CAR (■) or an irrelevant CAR (□). The arrow indicates time of retroviral transduction. (C) The expression of CAR on transgenic CTLs over time is shown. The number of stimulations after transduction is indicated. Bars represent average (± SD) for the 8 donors. (D) The immunophenotype of the EBV-CTLs was transduced with the CD30CAR (■) compared with EBV-CTLs transduced with an irrelevant CAR (□). ▩ show the phenotype of the CD30CAR+ CTLs stimulated with CD30+ tumor cells. Means (± SD) are shown for the 8 donors. (E) CD30CAR can be detected on both CD8+-transduced (left plot) and CD4+-transduced (right plot) EBV-CTLs.
Figure 2
CD30CAR-transduced EBV-CTLs specifically lyse CD30+ targets. (A) The results of a standard 51Cr-release assay of several CD30+ tumor-cell lines, at a CTL/tumor-cell ratio of 20:1, are shown. Bars represent the mean (± SD) of the EBV-CTLs generated from 8 donors and transduced with the CD30CAR (■) or an irrelevant CAR (□; P < .05). (B) Killing (shown is the percentage of lysis at 20:1 E/T ratio) of the CD30+ targets (■) by CD30CAR is inhibited by incubation with CD30 MAb (▒) but not by isotype control MAb (□) or by class I MHC MAb (▩), indicating that killing of CD30CAR is not MHC restricted. (*P < .05). Bars represent SD. (C) EBV-CTLs expressing the CD30CAR can eliminate CD30+ tumor cells in a long-term culture assay. EBV-CTLs obtained from healthy donors and transduced either with irrelevant CAR (left panels) or CD30CAR (right panels) were cocultured with the indicated CD30+ tumor-cell lines (ratio 5:1). After 5 to 7 days of culture, cells were collected and stained with CD3-PerCP and CD30-FITC to evaluate the growth of CD30+ tumor cells. No CD30+ cells were detectable after coculture with the CD30CAR+ EBV-CTLs, whereas CD30+ cells were detectable when tumor cells were cocultured with control CTLs. The phenotypes shown are representative of 4 performed experiments.
Figure 3
EBV-CTLs expressing the CD30CAR retain their ability to kill EBV+ tumor cells. (A)The mean (± SD) of 51Cr release from target cells exposed to EBV-CTLs from 8 donors transduced with an irrelevant CAR (left) and CD30CAR (right) is shown. CD30CAR+ CTLs lysed autologous LCLs (□) and the EBV−/CD30+ HD-derived cell line HDLM-2 (●; P < .05), whereas control CTLs showed significant lysis only of autologous LCLs. Autoreactivity was excluded by the absence of lysis of autologous PHA blasts (*). (B) CD30CAR+ CTLs (■) retain their killing activity against autologous LCLs and acquire the ability to kill allogeneic LCLs (* P < .05). As LCLs express CD30, this suggests that the observed lysis of allogeneic LCLs is mediated by the CAR. Bars indicate SD. Panel C shows that killing (at 20:1 E/T ratio) by CD30CAR+ CTLs of autologous LCLs (■) is not inhibited by incubation with class I MHC MAb (▩). This suggests that that killing of LCLs can still be mediated by the engagement of the CAR with the CD30 molecule expressed on LCLs. Bars indicate SD. (D) The expression of CD30 antigen on LCLs after depletion or enrichment for the CD30 molecule using immunomagnetic beads (MACS system) in 1 representative donor is shown. The percentage of specific 51Cr release at 40:1 E/T ratio of CTLs against CD30-depleted and CD30-selected autologous LCLs in 3 donors is shown in the bottom panel. Bars indicate SD.
Figure 4
EBV-CTLs redirected with CD30CAR retain their polyclonal EBV specificity. (A)The frequencies of tetramers recognizing lytic (BZLF1-RAK) or latent (EBNA3C-RPP and LMP2-CLG) EBV-associated antigens in control and transgenic EBV-CTLs generated from 3 different donors are shown. The bottom panels show that the same frequency of EBV-specific tetramers is maintained after transduction with CD30CAR. (B) The CD30CAR is also detectable on tetramer+ CTLs. (C) CFSE-labeled control (left panels) and CD30CAR+ CTLs (right panels) nonstimulated (top panels), stimulated with EBV+ (middle panels), or stimulated with CD30+ cells (bottom panels) are shown. After LCL stimulation (middle panels), both control and CD30CAR+ EBV-tetramer+ CTLs proliferate, as shown by decrease of CFSE+ cells. After stimulation with CD30+ cells (bottom panels), only CAR+ CTLs proliferate. (D) The frequency (mean ±SD) of T cells responding to EBV-specific peptides in control and CD30CAR+ EBV-CTLs from a representative donor, assessed by IFN-γ Elispot assay, is shown. Tetramer and Elispot analyses are representative of a total of 5 donors.
Figure 5
Reactivation of viral-specific T cells is not impaired in the presence of EBV-CTLs expressing the CD30CAR. Autologous EBV-CTLs engineered to express the CD30CAR were added to cultures of PBMCs stimulated to reactivate CMV- or adenovirus-specific CTLs. (A) The percentage of pp65-tetramer+ T cells generated in a representative donor by day 9 of culture in the presence of nontransduced EBV-CTLs (top plot), EBV-CTLs transduced with an irrelevant CAR (middle plot), or the CD30 CAR+ (bottom plot) are shown. (B) Cells from the coculture were stained with the goat anti–human IgG (H + L) Ab to demonstrate the continued presence of the CD30CAR+ CTLs throughout the culture. As expected, no CAR+ CTLs were detectable in cocultures where NT CTLs were added. In contrast, 21% and 25% CAR+ CTLs were detectable at the end of the cocultures where irrelevant CAR or CD30CAR+ CTLs were added, respectively. (C) The IFN-γ–specific Elispot assay of coculture from 2 representative donors is shown. Mean frequency (± SD) of IFN-γ–producing T cells in response to the CMV-specific peptides NLV and TRP is shown. (D) The percentage of adeno-tetramer+ T cells (shown is the analysis with the 2 available tetramers) is shown in the only donor whose viral-specific response was reduced when CD30CAR+ EBV-CTLs were added to the culture (see also Table 3).
Figure 6
EBV-CTLs generated from patients with HD can be grafted with a functional CD30CAR. EBV-CTL lines were expanded from PBMCs of 4 patients with HD. (A) The expression of CD30CAR on 2 representative CTL lines by flow cytometry using a goat anti–human IgG (H + L) Ab (solid line) is shown. The dotted line shows the isotype control. (B) The immunophenotype of EBV-CTLs generated from these 4 HD patients and transduced with the CD30CAR (■) compared with EBV-CTLs transduced with an irrelevant CAR (□) is shown. Mean and SD are shown. (C) The frequency of tetramers recognizing the lytic (BZLF1-RAK) EBV-associated antigen in EBV-CTLs generated from 1 of these patients is shown. The bottom panels show that the same frequency of EBV-specific tetramers is maintained after transduction with CD30CAR and that the CD30CAR is also detectable on tetramer+ T cells. (D) The killing of LCLs and CD30+ tumor cell lines in a standard 51Cr-release assay at a CTL/tumor cell ratio of 20:1 is shown. Bars represent the mean plus or minus the SD of the EBV-CTLs transduced with the CD30CAR (■) or an irrelevant CAR (□). CD30CAR+ CTLs lysed both autologous LCLs and CD30+ target cells, whereas control CTLs showed significant lysis only of autologous LCLs.
Figure 7
CD30CAR+ EBV-CTLs can control tumor growth in vivo while retaining their ability to migrate to EBV+ tumor and expand. To evaluate in vivo homing, NT EBV-CTLs or CTLs transduced with CD30CAR and sorted for transgene expression were injected intravenously in SCID mice implanted subcutaneously with autologous LCLs. Both NT and CAR+ EBV-CTLs were labeled with the eGFP-FFLuc gene to monitor their trafficking and expansion using an in vivo imaging system (Xenogen-IVIS Imaging System). (A) Circa 50% of CD30CAR+ EBV-CTLs, as assessed by the goat anti–human IgG (H + L) Ab, are expressing the FFLuc transgene as GFP+. (B) The signal of EBV-CTLs is localized to the EBV+ tumors and is elevated in mice receiving either control (top panels) or CD30CAR+ EBV-CTLs (bottom panels). (C) The bioluminescence fold expansion of CTLs at the tumor site is shown. To evaluate the contribution of costimulation by EBV antigen, EBV-CTLs transduced with irrelevant CAR or CD30CAR were injected intraperitoneally in SCID mice bearing EBV−/CD30+ L428 tumor that was transgenic for FFLuc. EBV-CTLs were transferred 7 days after tumor implant. Tumor growth was monitored using the in vivo imaging system. (D) By 7 days after CTL infusion, tumor growth measured as maximum photon/s/cm2/sr (p/s/cm2/sr) was significantly greater in mice receiving control CTLs (top panels) compared with mice receiving CD30CAR+ EBV-CTLs (middle panels). Persistence of tumor control can be observed in mice receiving CD30CAR+ EBV-CTLs and intraperitoneal injection of irradiated EBV-infected cells, which thus provide the appropriate costimulation (bottom panels). Panel E illustrates the results of 6 mice per group implanted with the CD30+ L428 cell line. Bars represent average of light emission (± SD) (P < .05).
Similar articles
- Epstein-Barr virus-specific human T lymphocytes expressing antitumor chimeric T-cell receptors: potential for improved immunotherapy.
Rossig C, Bollard CM, Nuchtern JG, Rooney CM, Brenner MK. Rossig C, et al. Blood. 2002 Mar 15;99(6):2009-16. doi: 10.1182/blood.v99.6.2009. Blood. 2002. PMID: 11877273 - Epstein-Barr virus (EBV)-specific cytotoxic T lymphocytes for the treatment of patients with EBV-positive relapsed Hodgkin's disease.
Roskrow MA, Suzuki N, Gan Yj, Sixbey JW, Ng CY, Kimbrough S, Hudson M, Brenner MK, Heslop HE, Rooney CM. Roskrow MA, et al. Blood. 1998 Apr 15;91(8):2925-34. Blood. 1998. PMID: 9531603 Clinical Trial. - Immunotherapy for Epstein-Barr virus-associated cancers in children.
Straathof KC, Bollard CM, Rooney CM, Heslop HE. Straathof KC, et al. Oncologist. 2003;8(1):83-98. doi: 10.1634/theoncologist.8-1-83. Oncologist. 2003. PMID: 12604735 Review. - Adoptive T-cell therapy for Epstein-Barr virus-positive Hodgkin's disease.
Huls MH, Rooney CM, Heslop HE. Huls MH, et al. Acta Haematol. 2003;110(2-3):149-53. doi: 10.1159/000072464. Acta Haematol. 2003. PMID: 14583675 Review.
Cited by
- CAR-T Cell Therapy for Classical Hodgkin Lymphoma.
Katsin M, Dormeshkin D, Meleshko A, Migas A, Dubovik S, Konoplya N. Katsin M, et al. Hemasphere. 2023 Nov 16;7(12):e971. doi: 10.1097/HS9.0000000000000971. eCollection 2023 Dec. Hemasphere. 2023. PMID: 38026793 Free PMC article. Review. - Chimeric Antigen Receptor Signaling Domains Differentially Regulate Proliferation and Native T Cell Receptor Function in Virus-Specific T Cells.
Omer B, Castillo PA, Tashiro H, Shum T, Huynh MTA, Cardenas M, Tanaka M, Lewis A, Sauer T, Parihar R, Lapteva N, Schmueck-Henneresse M, Mukherjee M, Gottschalk S, Rooney CM. Omer B, et al. Front Med (Lausanne). 2018 Dec 11;5:343. doi: 10.3389/fmed.2018.00343. eCollection 2018. Front Med (Lausanne). 2018. PMID: 30619856 Free PMC article. - Derivation of human T lymphocytes from cord blood and peripheral blood with antiviral and antileukemic specificity from a single culture as protection against infection and relapse after stem cell transplantation.
Micklethwaite KP, Savoldo B, Hanley PJ, Leen AM, Demmler-Harrison GJ, Cooper LJ, Liu H, Gee AP, Shpall EJ, Rooney CM, Heslop HE, Brenner MK, Bollard CM, Dotti G. Micklethwaite KP, et al. Blood. 2010 Apr 1;115(13):2695-703. doi: 10.1182/blood-2009-09-242263. Epub 2010 Jan 28. Blood. 2010. PMID: 20110422 Free PMC article. - Novel Immunotherapies for T Cell Lymphoma and Leukemia.
Ghione P, Moskowitz AJ, De Paola NEK, Horwitz SM, Ruella M. Ghione P, et al. Curr Hematol Malig Rep. 2018 Dec;13(6):494-506. doi: 10.1007/s11899-018-0480-8. Curr Hematol Malig Rep. 2018. PMID: 30317410 Free PMC article. Review. - Emerging Therapeutic Landscape of Peripheral T-Cell Lymphomas Based on Advances in Biology: Current Status and Future Directions.
Khan M, Samaniego F, Hagemeister FB, Iyer SP. Khan M, et al. Cancers (Basel). 2021 Nov 10;13(22):5627. doi: 10.3390/cancers13225627. Cancers (Basel). 2021. PMID: 34830782 Free PMC article. Review.
References
- Yung L, Linch D. Hodgkin's lymphoma. Lancet. 2003;361:943–951. - PubMed
- Aleman BM, Raemaekers JM, Tirelli U, et al. Involved-field radiotherapy for advanced Hodgkin's lymphoma. N Engl J Med. 2003;348:2396–2406. - PubMed
- Diehl V, Franklin J, Pfreundschuh M, et al. Standard and increased-dose BEACOPP chemotherapy compared with COPP-ABVD for advanced Hodgkin's disease. N Engl J Med. 2003;348:2386–2395. - PubMed
- Ng AK, Mauch PM. Late complications of therapy of Hodgkin's disease: prevention and management. Curr Hematol Rep. 2004;3:27–33. - PubMed
- Wu TC, Mann RB, Charache P, et al. Detection of EBV gene expression in Reed-Sternberg cells of Hodgkin's disease. Int J Cancer. 1990;46:801–804. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials