Unphosphorylated STAT3 accumulates in response to IL-6 and activates transcription by binding to NFkappaB - PubMed (original) (raw)
Unphosphorylated STAT3 accumulates in response to IL-6 and activates transcription by binding to NFkappaB
Jinbo Yang et al. Genes Dev. 2007.
Abstract
gp130-linked cytokines such as interleukin-6 (IL-6) stimulate the formation of tyrosine-phosphorylated signal transducer and activator of transcription 3 (P-STAT3), which activates many genes, including the STAT3 gene itself. The resulting increase in the concentration of unphosphorylated STAT3 (U-STAT3) drives a second wave of expression of genes such as RANTES, IL6, IL8, MET, and MRAS that do not respond directly to P-STAT3. Thus, U-STAT3 sustains cytokine-dependent signaling at late times through a mechanism completely distinct from that used by P-STAT3. Many U-STAT3-responsive genes have kappaB elements that are activated by a novel transcription factor complex formed when U-STAT3 binds to unphosphorylated NFkappaB (U-NFkappaB), in competition with IkappaB. The U-STAT3/U-NFkappaB complex accumulates in the nucleus with help from the nuclear localization signal of STAT3, activating a subset of kappaB-dependent genes. Additional genes respond to U-STAT3 through an NFkappaB-independent mechanism. The role of signal-dependent increases in U-STAT3 expression in regulating gene expression is likely to be important in physiological responses to gp130-linked cytokines and growth factors that activate STAT3, and in cancers that have constitutively active P-STAT3.
Figures
Figure 1.
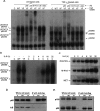
The ability of U-STAT3 to regulate the RANTES promoter depends on a κB element. (A) Western and Northern analyses for STAT3 and RANTES expression in hTERT-HME1-derived cells. The cells were infected with retroviral constructs and stable pools were selected with G418. (C) hTERT-HME1 control cells; (WT) hTERT-HME1 cells expressing a high level of wild-type STAT3; (YF) hTERT-HME1 cells expressing a high level of Y705F-STAT3. Total cell lysates and total RNAs were analyzed. (B) Basal transcriptional activity of the human RANTES promoter in hTERT-HME1 cells. Luciferase constructs containing 5′- or 3′-deletions between bases −974 and −1 of the promoter were cotransfected with the pCH110 control plasmid and the cells were harvested 48 h later. The luciferase activity in each cell extract was normalized to the level of β-galactosidase activity (from pCH110) in the same extract. Values are means of triplicate determinations, and the bars show one standard error of the mean. (C) Inducible activity of human RANTES promoter fragments in YF cells. The reporter constructs were cotransfected with pCH110. The activities shown are relative to the activity of each fragment in hTERT-HME1 control cells. Values are means of triplicate determinations, and the bars show one standard error of the mean. (D) Inducible activity of promoter mutations in YF cells. The reporter constructs, containing mutations of individual promoter elements (marked by ×) of the 220-base-pair promoter fragment were transfected into the cells. Luciferase activities were determined and calculated relative to the values obtained in control cells as in C. (E) Y705F-STAT3 and p65 cooperate to drive the RANTES promoter. hTERT-HME1 cells were cotransfected with the pGL2-220 plasmid, in which the RANTES −1 to −220 promoter fragment drives luciferase expression, and pCH110, with or without pcDNA3.1-Y705F-STAT3 or pcDNA3.1-p65, expression plasmids for Y705F-STAT3 and p65, respectively. The cells, harvested 48 h later, were analyzed for luciferase activities, as described above. The reporter activities were normalized to activities in cells without cotransfection of p65 or Y705F-STAT3. Values are means of triplicate determinations, and the bars show one standard error of the mean. (F) U-STAT3 and p65 cooperate to activate the RANTES gene. hTERT-HME1 cells were transfected transiently with pcDNA3.1-p65 and/or pcDNA3.1-STAT3. After 48 h, total RNAs were isolated and analyzed by the Northern method.
Figure 2.
U-STAT3 binds to U-NFκB. (A) DNA-binding assays. The EMSAs shown were performed with whole-cell extracts. Assays with nuclear extracts (not shown) gave similar results. (C) hTERT-HME1 control cells. A DNA fragment of the human RANTES promoter, bases −58 to −29, containing a κB element, was used as the labeled probe. (Lanes 1–8) Extracts of untreated cells: control cells (lane 1), WT cells (lane 2), YF cells (lane 3), and supershifts obtained with extracts of YF cells following addition of antibodies directed against p65, p50, STAT3, IκB, or c-Myc (lanes 4–8). (Lanes 9–16) Same as lanes 1–8 except that the extracts are from cells treated with TNF-α for 4 h. (B) EMSAs. Whole-cell extracts were made from hTERT-HME1 cells, untreated or treated with IL-6. The probe was same as in A. (C) Northern analysis. Total RNAs (20 μg per lane) from hTERT-HME1 cells untreated or treated with IL-6 were analyzed by the Northern method. (D,E) STAT3 binds to p65, p50, and p105 but not to IκB. STAT3 was immunoprecipitated from whole-cell extracts of the cells shown in Figure 1A by using anti-Flag M2 beads. Western analyses were performed to detect p65, p50, and IκB.
Figure 3.
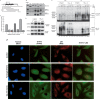
The SH2 and NLS domains of STAT3 are required for interaction with p65 and p50 and for up-regulation of the RANTES promoter. (A) STAT3 domains and deletion constructs. (B) PC3 cells were transfected with expression constructs for N- and C-terminal deletions of STAT3 and, 48 h later, whole-cell lysates were prepared and assayed by coimmunoprecipitation with anti-p65 or anti-p50 and by the Western method with anti-STAT3. Three different antibodies that react with the N-terminal, C-terminal, and middle portions of STAT3 were used. (C) Expression constructs for N- and C-terminal deletions of STAT3 were cotransfected into PC3 cells with pGL2-220 and pCH110, and the cells were harvested for luciferase assays 48 h later. (D) Northern analysis. Total RNAs (20 μg per lane) from hTERT-HME1 cells untreated or treated with IL-6 were analyzed by the Northern method. (E) DNA-binding assays. hTERT-HME1 cells expressing a high level of full-length STAT3 or 162–770 truncated STAT3 were untreated or treated with TNF-α for 4 h. EMSAs were performed with cytoplasmic and nuclear fractions. A DNA fragment of the human RANTES promoter, bases −58 to −29, containing a κB element, was used as the labeled probe. Assays were performed by adding equal amounts of proteins. (C) hTERT-HME1 control cells; (S3) hTERT-HME1 cells expressing a high level of STAT3. (F) hTERT-HME1 cells expressing a high level of full-length or 162–770 truncated STAT3 were grown on cover slips and stained with primary antibodies directed against STAT3 and p65. Following treatment with DAPI (blue nuclear stain) and fluorescent secondary antibodies for STAT3 (green) and p65 (red), the cells were examined by using confocal microscopy. The yellow pixels in the composite image demonstrate the close association of the two proteins.
Figure 4.
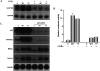
Inhibition of NFκB decreases RANTES gene expression in response to U-STAT3. (A) hTERT-HME1-derived cells were transfected transiently with the pcDNA3.1-mIκBα construct, which encodes the NFκB superrepressor and, 48 h later, total RNAs were isolated and analyzed. (B) The RANTES promoter-driven luciferase reporter construct pGL2-220 was transfected with pCH110, with or without pcDNA3.1-mIκBα, into hTERT-HME1-derived cells and, 48 h later, luciferase assays were performed. (C) hTERT-HME1-derived cells were transfected transiently with a siRNA directed against p65 and, 24 h later, the cells were transfected again as in A. The cells were harvested after 48 h more and total RNA was extracted. All of the mRNAs shown were assayed on the same Northern transfer.
Figure 5.
Comparison of genes induced by high-level expression of Y705F-STAT3 with those induced by treatment with TNF-α. hTERT-HME1 control cells or YF cells were treated with 50 ng/mL TNF-α for 4 h or were untreated. Total RNAs were isolated and analyzed by using the CodeLink gene chip system. Genes with a more than threefold change in expression, compared with expression in untreated hTERT-HME1 cells, were scored. (A) Comparison of the genes expressed in response to a high level of Y705F-STAT3 or treatment with TNF-α. (B) Northern analysis of gene expression.
Figure 6.
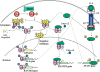
Interactions between the STAT3 and NFκB pathways. U-STAT3, induced to a high level due to activation of the STAT3 gene in response to ligands such as IL-6, competes with IκB for p65/p50. The U-STAT3:U-NFκB complex activates the RANTES promoter plus a subset of other promoters that have κB elements. U-STAT3 also drives the expression of some genes that do not have κB elements, by an unknown mechanism (not shown). The κB element of the IL6 gene is driven by canonical NFκB signaling in response to ligands such as TNF-α or IL-1, setting up the positive feedback loop that is driven by the activation of STAT3 in response to secreted IL-6, leading to an increased level of U-STAT3 that sustains the activation of genes such as RANTES. (Imp-α3) Importin-α3.
Similar articles
- Unraveling the unphosphorylated STAT3-unphosphorylated NF-κB pathway in loss of function STAT3 Hyper IgE syndrome.
Karim A, Garg R, Saikia B, Tiwari A, Sahu S, Malhotra M, Minz RW, Rawat A, Singh S, Suri D. Karim A, et al. Front Immunol. 2024 Aug 20;15:1332817. doi: 10.3389/fimmu.2024.1332817. eCollection 2024. Front Immunol. 2024. PMID: 39229272 Free PMC article. - Novel roles of unphosphorylated STAT3 in oncogenesis and transcriptional regulation.
Yang J, Chatterjee-Kishore M, Staugaitis SM, Nguyen H, Schlessinger K, Levy DE, Stark GR. Yang J, et al. Cancer Res. 2005 Feb 1;65(3):939-47. Cancer Res. 2005. PMID: 15705894 - Molecular cross-talk between the NFkappaB and STAT3 signaling pathways in head and neck squamous cell carcinoma.
Squarize CH, Castilho RM, Sriuranpong V, Pinto DS Jr, Gutkind JS. Squarize CH, et al. Neoplasia. 2006 Sep;8(9):733-46. doi: 10.1593/neo.06274. Neoplasia. 2006. PMID: 16984731 Free PMC article. - The functions of signal transducers and activators of transcriptions 1 and 3 as cytokine-inducible proteins.
Cheon H, Yang J, Stark GR. Cheon H, et al. J Interferon Cytokine Res. 2011 Jan;31(1):33-40. doi: 10.1089/jir.2010.0100. Epub 2010 Dec 19. J Interferon Cytokine Res. 2011. PMID: 21166594 Free PMC article. Review. - Pivotal Importance of STAT3 in Protecting the Heart from Acute and Chronic Stress: New Advancement and Unresolved Issues.
Zouein FA, Altara R, Chen Q, Lesnefsky EJ, Kurdi M, Booz GW. Zouein FA, et al. Front Cardiovasc Med. 2015 Nov 30;2:36. doi: 10.3389/fcvm.2015.00036. eCollection 2015. Front Cardiovasc Med. 2015. PMID: 26664907 Free PMC article. Review.
Cited by
- Targeting the interleukin-6/Jak/stat pathway in human malignancies.
Sansone P, Bromberg J. Sansone P, et al. J Clin Oncol. 2012 Mar 20;30(9):1005-14. doi: 10.1200/JCO.2010.31.8907. Epub 2012 Feb 21. J Clin Oncol. 2012. PMID: 22355058 Free PMC article. Review. - A New STAT3-binding Partner, ARL3, Enhances the Phosphorylation and Nuclear Accumulation of STAT3.
Togi S, Muromoto R, Hirashima K, Kitai Y, Okayama T, Ikeda O, Matsumoto N, Kon S, Sekine Y, Oritani K, Matsuda T. Togi S, et al. J Biol Chem. 2016 May 20;291(21):11161-71. doi: 10.1074/jbc.M116.724849. Epub 2016 Apr 5. J Biol Chem. 2016. PMID: 27048653 Free PMC article. - Asymmetric post-translational modifications regulate the nuclear translocation of STAT3 homodimers in response to leukemia inhibitory factor.
Diallo M, Pimenta C, Murtinheira F, Martins-Alves D, Pinto FR, da Costa AA, Letra-Vilela R, Martin V, Rodriguez C, Rodrigues MS, Herrera F. Diallo M, et al. Cell Oncol (Dordr). 2024 Jun;47(3):1065-1070. doi: 10.1007/s13402-023-00911-9. Epub 2023 Dec 27. Cell Oncol (Dordr). 2024. PMID: 38150153 Free PMC article. - A novel tumor-promoting mechanism of IL6 and the therapeutic efficacy of tocilizumab: Hypoxia-induced IL6 is a potent autophagy initiator in glioblastoma via the p-STAT3-MIR155-3p-CREBRF pathway.
Xue H, Yuan G, Guo X, Liu Q, Zhang J, Gao X, Guo X, Xu S, Li T, Shao Q, Yan S, Li G. Xue H, et al. Autophagy. 2016 Jul 2;12(7):1129-52. doi: 10.1080/15548627.2016.1178446. Epub 2016 May 10. Autophagy. 2016. PMID: 27163161 Free PMC article. - STAT3 and NF-κB cooperatively control in vitro spontaneous apoptosis and poor chemo-responsiveness in patients with chronic lymphocytic leukemia.
Liu FT, Jia L, Wang P, Wang H, Farren TW, Agrawal SG. Liu FT, et al. Oncotarget. 2016 May 31;7(22):32031-45. doi: 10.18632/oncotarget.8672. Oncotarget. 2016. PMID: 27074565 Free PMC article.
References
- Agrawal A., Cha-Molstad H., Samols D., Kushner I., Cha-Molstad H., Samols D., Kushner I., Samols D., Kushner I., Kushner I. Overexpressed nuclear factor-κB can participate in endogenous C-reactive protein induction, and enhances the effects of C/EBPβ and signal transducer and activator of transcription-3. Immunology. 2003;108:539–547. - PMC - PubMed
- Akira S., Nishio Y., Inoue M., Wang X.J., Wei S., Matsusaka T., Yoshida K., Sudo T., Naruto M., Kishimoto T., Nishio Y., Inoue M., Wang X.J., Wei S., Matsusaka T., Yoshida K., Sudo T., Naruto M., Kishimoto T., Inoue M., Wang X.J., Wei S., Matsusaka T., Yoshida K., Sudo T., Naruto M., Kishimoto T., Wang X.J., Wei S., Matsusaka T., Yoshida K., Sudo T., Naruto M., Kishimoto T., Wei S., Matsusaka T., Yoshida K., Sudo T., Naruto M., Kishimoto T., Matsusaka T., Yoshida K., Sudo T., Naruto M., Kishimoto T., Yoshida K., Sudo T., Naruto M., Kishimoto T., Sudo T., Naruto M., Kishimoto T., Naruto M., Kishimoto T., Kishimoto T. Molecular cloning of APRF, a novel IFN-stimulated gene factor 3 p91-related transcription factor involved in the gp130-mediated signaling pathway. Cell. 1994;77:63–71. - PubMed
- Bacon K.B., Premack B.A., Gardner P., Schall T.J., Premack B.A., Gardner P., Schall T.J., Gardner P., Schall T.J., Schall T.J. Activation of dual T cell signaling pathways by the chemokine RANTES. Science. 1995;269:1727–1730. - PubMed
- Battle T.E., Frank D.A., Frank D.A. The role of STATs in apoptosis. Curr. Mol. Med. 2002;2:381–392. - PubMed
- Birbach A., Gold P., Binder B.R., Hofer E., de Martin R., Schmid J.A., Gold P., Binder B.R., Hofer E., de Martin R., Schmid J.A., Binder B.R., Hofer E., de Martin R., Schmid J.A., Hofer E., de Martin R., Schmid J.A., de Martin R., Schmid J.A., Schmid J.A. Signaling molecules of the NF-κB pathway shuttle constitutively between cytoplasm and nucleus. J. Biol. Chem. 2002;277:10842–10851. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous