Treadmilling of a prokaryotic tubulin-like protein, TubZ, required for plasmid stability in Bacillus thuringiensis - PubMed (original) (raw)
Comparative Study
. 2007 Jun 1;21(11):1340-52.
doi: 10.1101/gad.1546107. Epub 2007 May 17.
Affiliations
- PMID: 17510284
- PMCID: PMC1877747
- DOI: 10.1101/gad.1546107
Comparative Study
Treadmilling of a prokaryotic tubulin-like protein, TubZ, required for plasmid stability in Bacillus thuringiensis
Rachel A Larsen et al. Genes Dev. 2007.
Abstract
Prokaryotes rely on a distant tubulin homolog, FtsZ, for assembling the cytokinetic ring essential for cell division, but are otherwise generally thought to lack tubulin-like polymers that participate in processes such as DNA segregation. Here we characterize a protein (TubZ) from the Bacillus thuringiensis virulence plasmid pBtoxis, which is a member of the tubulin/FtsZ GTPase superfamily but is only distantly related to both FtsZ and tubulin. TubZ assembles dynamic, linear polymers that exhibit directional polymerization with plus and minus ends, movement by treadmilling, and a critical concentration for assembly. A point mutation (D269A) that alters a highly conserved catalytic residue within the T7 loop completely eliminates treadmilling and allows the formation of stable polymers at a much lower protein concentration than the wild-type protein. When expressed in trans, TubZ(D269A) coassembles with wild-type TubZ and significantly reduces the stability of pBtoxis, demonstrating a direct correlation between TubZ dynamics and plasmid maintenance. The tubZ gene is in an operon with tubR, which encodes a putative DNA-binding protein that regulates TubZ levels. Our results suggest that TubZ is representative of a novel class of prokaryotic cytoskeletal proteins important for plasmid stability that diverged long ago from the ancient tubulin/FtsZ ancestor.
Figures
Figure 1.
Phylogenetic analysis of the tubulin superfamily. (A) Phylogenetic tree showing the relationship between tubulins (blue), FtsZ (green), and TubZ (Archaea, pink; Bacillus, red). The tree was calculated using the neighbor-joining method, and bootstrap values representing confidence levels are indicated for select branches. Sequence abbreviations are listed below. Accession numbers are provided in the Materials and Methods and Supplemental Material. For each of the proteins from Bacillus plasmids, the name of the plasmid as well as the ORF number is noted on parentheses. (B) Alignment of conserved motifs for selected sequences. Each motif (loops T1, T4, T6, T7) as previously annotated (Nogales et al. 1998a) is shown with universally conserved residues indicated in red, residues conserved in FtsZ indicated in green, and residues conserved in tubulin indicated in blue. Sequence abbreviations are as follows: (Ec) E. coli FtsZ; (Bs) B. subtilis FtsZ; (Hm1) Haloarcula marismortui FtsZ1; (Hm4) H. marismortui FtsZ4; (Hh4) Halobacterium NRC-1 FtsZ4; (Hm5) H. marismortui FtsZ5; (Hh5) Halobacterium NRC-1 FtsZ5; (Bm) B. megaterium pBM400 orf44; (Bc) B. cereus pBc218 orf139; (Ba) B. anthracis pXO1 orf45; (Bt) B. thuringiensis pBtoxis orf156; (SsA) Sus scrofa α tubulin; (SsB) S. scrofa β tubulin; (HsG) Homo sapiens γ tubulin; (HsE) H. sapiens ε tubulin; (HsD) H. sapiens δ tubulin.
Figure 2.
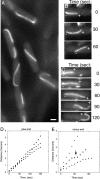
TubZ-GFP assembles dynamic polymers in B. thuringiensis. (A) Fluorescence microscopy of B. thuringiensis expressing TubZ-GFP. Bar, 1 μm. (B,C) Time-lapse microscopy of TubZ-GFP movement. The plus (+) and minus (−) ends of each filament are noted to indicate the direction of movement. Images at 30-sec intervals are presented. (D) Rate of plus end movement versus time. The different points (squares, triangles, circles) represent measurements for three individual filaments. (E) Rate of minus end movement versus time. The arrow indicates a pause in this rate, likely due to a pause in depolymerization. The different points (squares, triangles, circles) represent measurements for three individual filaments.
Figure 3.
TubZ-GFP assembles dynamic polymers in E. coli. Time-lapse microscopy of E. coli strain JP2167 expressing TubZ-GFP with time points indicated in seconds. The plus (+) end indicates the forward direction of movement. Bar, 1 μm. (A–C) Single filaments translocate within the cell. (D) Two filaments move simultaneously in opposite directions. (E) FRAP analysis demonstrates that translocation occurs by treadmilling. A prebleach image and post-bleach images collected at 12-sec intervals are shown. For clarity, the example shown is a cell treated with cephalexin. (F) Rate of plus end movement versus time for three individual filaments (squares, triangles, circles). (G) Rate of minus end movement versus time for two individual filaments (triangles, circles). (H) Model for the treadmilling mechanism of TubZ movement in vivo. New subunits assemble at the plus end (●) while subunits are removed from the minus end (○).
Figure 4.
Assembly dynamics of TubZ-GFP filaments in cephalexin-treated cells. Time-lapse microscopy of E. coli strain JP2167 expressing TubZ-GFP after incubation with 10 μg/mL cephalexin for 1 h with time points indicated in seconds. (A) Elongation and movement of TubZ-GFP. The plus (+) and minus (−) ends are marked by white triangles. The plus (+) end of the filament grew at a rate of 0.98 μm/min while the minus (−) end remained stationary. (Right) A second polymer in the cell translocated around the cell pole and continued movement in the opposite direction. (B) Movement of two filaments in opposite directions. Plus (+) and minus (−) ends of each are noted. (C) Rapid disassembly of TubZ-GFP filaments. A filament, with the ends noted by white triangles, rapidly shrank apparently from both ends after cells were incubated on a glass slide without nutrients. Bar, 1 μm.
Figure 5.
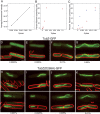
TubZ-GFP has a critical concentration for assembly in B. thuringiensis. (A) Average fluorescence intensity versus xylose concentration for expression of wild-type TubZ-GFP. Protein expression was quantified by measuring fluorescence intensity of individual cells expressed per unit area. Averages (n = 100) for each xylose concentration are plotted. (B) Percentage of filament-containing cells plotted against xylose concentration for JP2209 expressing wild-type TubZ-GFP. Values from the lower range (0%–0.02%) are replotted in C for clarity. (C) Percentage of filament-containing cells plotted against xylose concentration for JP2209 expressing wild-type TubZ-GFP (red) and for JP2217 expressing TubZ(D269A)-GFP (blue). A narrow range of xylose concentrations (0%–0.02%) is plotted to compare the difference between the wild type and the mutant. (D–G) Images of JP2209 expressing wild-type TubZ-GFP after growth in 0.0025% (D), 0.005% (E), 0.01% (F), and 0.02% (G) xylose. Bar, 1 μm. (H–K) Images of JP2217 expressing TubZ(D269A)-GFP after growth in 0.001% (D), 0.0025% (E), 0.005% (F), and 0.01% (G) xylose.
Figure 6.
TubZ(D269A)-GFP forms irregular, static filaments. Polymer structure and dynamics were examined for TubZ(D269A)-GFP induced with 0.0025% xylose in strain JP2217. At this xylose concentration, filaments of the mutant are similar in length to wild-type TubZ-GFP. (A) A comparison of the uniformity of filaments in wild-type (left panel) versus TubZ(D269A) (right panel). Both filaments are within the focal plane. A three-dimensional graph showing the pixel intensity for each image is shown to highlight the differences between the two. (B) Rates of movement of TubZ(D269A)-GFP of two filaments (squares and diamonds) are presented as distance traveled versus time. Rate of movement of a wild-type TubZ-GFP filament (circles) is included for comparison. (C) Time-lapse microscopy of a TubZ(D269A)-GFP filament with time points indicated in seconds. Bar 1 μm. (D,E) FRAP analysis of TubZ(D269A)-GFP filaments. A small region (indicated by the red circle) was bleached with an argon laser and images captured at 40-sec intervals post-bleach are presented.
Figure 7.
Disruption of TubZ dynamics by overexpression or by mutation affects the stability of pBtoxis. (A–C) Colony PCR (n = 100) of strains expressing either wild-type TubZ-GFP or TubZ(D269A)-GFP after sporulation in the presence of the indicated concentrations of xylose was performed to detect the presence of pBtoxis. The larger PCR product (labeled “pBt”) was amplified from pBtoxis and the smaller PCR product (labeled “c”) was amplified from the B. thuringiensis chromosome as a control. (A) Representative agarose gels from PCR analysis of JP2219 (expressing wild-type TubZ-GFP). (B) Representative agarose gels from PCR analysis of JP2227 [expressing TubZ(D269A)-GFP]. (C) Summary of results from the PCR analysis. (D) TubZ(D269A) coassembles with wild-type TubZ-GFP. The left panel depicts the predicted behavior of the wild-type and mutant subunits when expressed in the same cell. The right panel is a representative image from fluorescent microscopy of JP2241 showing GFP alone (top) and GFP with FM4-64-stained membranes (bottom). Bar, 1 μm.
Figure 8.
Regulation of TubZ by TubR. TubR was expressed in trans from an arabinose-inducible promoter in E. coli, and the effect on TubZ-GFP expression was determined by Western blotting using anti-GFP antibody. (Top) A diagram of the tubRZ operon on plasmids pRL165 (wild type) and pCUS12 (Δ_tubR_). (Bottom) Western blotting showing the relative amounts of TubZ-GFP expressed from either pRL165 or pCUS12 in the presence or absence of TubR expressed in trans from pRL186 (PBAD-tubR). TubZ-GFP expression in the presence of plasmid pBAD33 (PBAD) is included as a control. TubZ-GFP bands were quantified and values were adjusted for the amount of cells loaded on the gel. (Lane 5) Wild-type levels were arbitrarily set to 1.0 and the relative levels of other samples are noted below each band.
Similar articles
- Reconstitution of a prokaryotic minus end-tracking system using TubRC centromeric complexes and tubulin-like protein TubZ filaments.
Fink G, Löwe J. Fink G, et al. Proc Natl Acad Sci U S A. 2015 Apr 14;112(15):E1845-50. doi: 10.1073/pnas.1423746112. Epub 2015 Mar 30. Proc Natl Acad Sci U S A. 2015. PMID: 25825718 Free PMC article. - Plasmid protein TubR uses a distinct mode of HTH-DNA binding and recruits the prokaryotic tubulin homolog TubZ to effect DNA partition.
Ni L, Xu W, Kumaraswami M, Schumacher MA. Ni L, et al. Proc Natl Acad Sci U S A. 2010 Jun 29;107(26):11763-8. doi: 10.1073/pnas.1003817107. Epub 2010 Jun 4. Proc Natl Acad Sci U S A. 2010. PMID: 20534443 Free PMC article. - In vitro assembly studies of FtsZ/tubulin-like proteins (TubZ) from Bacillus plasmids: evidence for a capping mechanism.
Chen Y, Erickson HP. Chen Y, et al. J Biol Chem. 2008 Mar 28;283(13):8102-9. doi: 10.1074/jbc.M709163200. Epub 2008 Jan 15. J Biol Chem. 2008. PMID: 18198178 Free PMC article. - Tubulin-Like Proteins in Prokaryotic DNA Positioning.
Fink G, Aylett CHS. Fink G, et al. Subcell Biochem. 2017;84:323-356. doi: 10.1007/978-3-319-53047-5_11. Subcell Biochem. 2017. PMID: 28500531 Review. - Evolution of the cytoskeleton.
Erickson HP. Erickson HP. Bioessays. 2007 Jul;29(7):668-77. doi: 10.1002/bies.20601. Bioessays. 2007. PMID: 17563102 Free PMC article. Review.
Cited by
- The IntXO-PSL Recombination System Is a Key Component of the Second Maintenance System for Bacillus anthracis Plasmid pXO1.
Pomerantsev AP, Rappole C, Chang Z, Chahoud M, Leppla SH. Pomerantsev AP, et al. J Bacteriol. 2016 Jun 27;198(14):1939-1951. doi: 10.1128/JB.01004-15. Print 2016 Jul 15. J Bacteriol. 2016. PMID: 27137503 Free PMC article. - Unique Properties of the Alpha-Helical DNA-Binding Protein KfrA Encoded by the IncU Incompatibility Group Plasmid RA3 and Its Host-Dependent Role in Plasmid Maintenance.
Lewicka E, Mitura M, Steczkiewicz K, Kieracinska J, Skrzynska K, Adamczyk M, Jagura-Burdzy G. Lewicka E, et al. Appl Environ Microbiol. 2021 Jan 4;87(2):e01771-20. doi: 10.1128/AEM.01771-20. Print 2021 Jan 4. Appl Environ Microbiol. 2021. PMID: 33097508 Free PMC article. - Maintenance of multipartite genome system and its functional significance in bacteria.
Misra HS, Maurya GK, Kota S, Charaka VK. Misra HS, et al. J Genet. 2018 Sep;97(4):1013-1038. J Genet. 2018. PMID: 30262715 Review. - The parABSm system is involved in megaplasmid partitioning and genome integrity maintenance in Thermus thermophilus.
Li H, Xu L, Li X. Li H, et al. G3 (Bethesda). 2023 Apr 11;13(4):jkad038. doi: 10.1093/g3journal/jkad038. G3 (Bethesda). 2023. PMID: 36786449 Free PMC article. - Engineering spatiotemporal organization and dynamics in synthetic cells.
Groaz A, Moghimianavval H, Tavella F, Giessen TW, Vecchiarelli AG, Yang Q, Liu AP. Groaz A, et al. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2021 May;13(3):e1685. doi: 10.1002/wnan.1685. Epub 2020 Nov 21. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2021. PMID: 33219745 Free PMC article. Review.
References
- Adachi S., Hori K., Hiraga S., Hori K., Hiraga S., Hiraga S. Subcellular positioning of F plasmid mediated by dynamic localization of SopA and SopB. J. Mol. Biol. 2006;356:850–863. - PubMed
- Addinall S.G., Holland B., Holland B. The tubulin ancestor, FtsZ, draughtsman, designer and driving force for bacterial cytokinesis. J. Mol. Biol. 2002;318:219–236. - PubMed
- Amos L.A., van den Ent F., Lowe J., van den Ent F., Lowe J., Lowe J. Structural/functional homology between the bacterial and eukaryotic cytoskeletons. Curr. Opin. Cell Biol. 2004;16:24–31. - PubMed
- Becker E., Herrera N.C., Gunderson F.Q., Derman A.I., Dance A.L., Sims J., Larsen R.A., Pogliano J., Herrera N.C., Gunderson F.Q., Derman A.I., Dance A.L., Sims J., Larsen R.A., Pogliano J., Gunderson F.Q., Derman A.I., Dance A.L., Sims J., Larsen R.A., Pogliano J., Derman A.I., Dance A.L., Sims J., Larsen R.A., Pogliano J., Dance A.L., Sims J., Larsen R.A., Pogliano J., Sims J., Larsen R.A., Pogliano J., Larsen R.A., Pogliano J., Pogliano J. DNA segregation by the bacterial actin AlfA during Bacillus subtilis growth and development. EMBO J. 2006;25:5919–5931. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources