Gliogenesis and glial pathology in depression - PubMed (original) (raw)
Review
Gliogenesis and glial pathology in depression
G Rajkowska et al. CNS Neurol Disord Drug Targets. 2007 Jun.
Abstract
Recent research has changed the perception of glia from being no more than silent supportive cells of neurons to being dynamic partners participating in brain metabolism and communication between neurons. This discovery of new glial functions coincides with growing evidence of the involvement of glia in the neuropathology of mood disorders. Unanticipated reductions in the density and number of glial cells are reported in fronto-limbic brain regions in major depression and bipolar illness. Moreover, age-dependent decreases in the density of glial fibrillary acidic protein (GFAP) - immunoreactive astrocytes and levels of GFAP protein are observed in the prefrontal cortex of younger depressed subjects. Since astrocytes participate in the uptake, metabolism and recycling of glutamate, we hypothesize that an astrocytic deficit may account for the alterations in glutamate/GABA neurotransmission in depression. Reductions in the density and ultrastructure of oligodendrocytes are also detected in the prefrontal cortex and amygdala in depression. Pathological changes in oligodendrocytes may be relevant to the disruption of white matter tracts in mood disorders reported by diffusion tensor imaging. Factors such as stress, excess of glucocorticoids, altered gene expression of neurotrophic factors and glial transporters, and changes in extracellular levels of neurotransmitters released by neurons may modify glial cell number and affect the neurophysiology of depression. Therefore, we will explore the role of these events in the possible alteration of glial number and activity, and the capacity of glia as a promising new target for therapeutic medications. Finally, we will consider the temporal relationship between glial and neuronal cell pathology in depression.
Figures
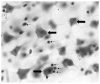
Fig. (1)
Photomicrograph showing a Nissl stained 40 μm thick section from the dorsolateral prefrontal cortex of human postmortem brain. Large black arrows indicate neuronal cell bodies. Small black arrows show the location of glial cells and the white arrows display examples of perineuronal oligodendrocytes. The image was taken using the 40x immersion oil objective of a Nikon Eclipse E600 microscope.
Fig. (2)
Photomicrographs displaying the different types of immunopositive neuroglia in the prefrontal cortex of a psychiatrically normal human subject. Images A and B show two types of astroglia both immunostained with an anti-GFAP (glial fibrillary acid protein) antibody which is a marker for reactive astroglia. Protoplasmic astrocytes (A) are found mainly in cortical gray matter, and fibrous astrocytes (B) are found mainly in cortical white matter. Image C displays oligodendrocytes in cortical white matter which are immunostained with anti CNPase antibody, a marker for mature oligodendrocytes. Image D illustrates microglia in white matter immunostained for anti LFA-1 antibody, a marker of active microglia. All photomicrographs were taken with the 20x objective of a Nikon Eclipse E600 microscope.
Fig. (3)
Photomicrographs (A) of the distribution of glial fibrillary acidic protein (GFAP) immunoreactivity in the prefrontal cortex of a pair of young subjects. The top image displays a control subject (27 years old,) with the bottom image illustrating a subject diagnosed with major depressive disorder (MDD) (32 years old,). Note a much reduced GFAP immunolabeling in the MDD subject. Blot immunolabeling of GFAP in depicted in part B of the figure. Two pairs of subjects, control and MDD, display two bands identified as GFAP (50kDa) and actin (42kDa). For each pair the ages of the subjects are indicated in parentheses. Note that GFAP levels from the younger depressed subject are markedly lower than those of the young control subject, whereas, the older depressed subject has more GFAP than its matched control. Scatter plot (C) illustrates the significant positive correlation between areal fraction of GFAP-immunoreactive astrocytes and age in the prefrontal cortex of subjects with MDD. Note that lower values for GFAP areal fraction were found in younger depressed subjects as opposed to older subjects with depression.
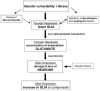
Fig. (4)
Hypothetical scheme of the progression of cell pathology in depression from young adulthood to old age This scheme incorporates the relationships between cellular, glutamate, glucocorticoid, and neurotrophic factor alterations in depression. It is proposed that a combination of genetic and environmental (e.g. stress) factors at the early stages of depressive illness could lead initially to the pathology of glial cells, and consequently to the pathology of neurons later in life as depressive illness progresses. Among the factors contributing to early glial pathology might be stress-related elevations in glucocorticoids and/or a deficiency in neurotrophic and angiogenic factors. It is further proposed that the loss of glial cells at early stages of depression may lead to decreased expression of glial glutamate transporters and a concomitant reduction in the uptake of glutamate from the synaptic cleft. As a consequence, an excess of extracellular glutamate may contribute to neuronal damage or neuronal death as depressed subjects get older. In light of the ability of glia to proliferate in response to neuronal injury, it could be hypothesized that neuronal injury in depression could result in glial proliferation in elderly subjects with depression. However, other factors (vascular lesions, age-related atrophy of neurons and fiber tracts, and medical comorbidity) in addition to glial deficits could contribute to neuronal pathology in older depressed subjects, particularly in those with the first onset of depression in late-life. Thus, the box “Vascular Lesions” mostly represents a new cohort of patients with late onset depression who might not have had glial pathology earlier in life (for further details please refer to the section: “Model of Glial/Neuronal Cell Pathology in Depression”).
Similar articles
- Region specific decrease in glial fibrillary acidic protein immunoreactivity in the brain of a rat model of depression.
Gosselin RD, Gibney S, O'Malley D, Dinan TG, Cryan JF. Gosselin RD, et al. Neuroscience. 2009 Mar 17;159(2):915-25. doi: 10.1016/j.neuroscience.2008.10.018. Epub 2008 Nov 1. Neuroscience. 2009. PMID: 19000745 - Glial cells as key elements in the pathophysiology and treatment of bipolar disorder.
Keshavarz M. Keshavarz M. Acta Neuropsychiatr. 2017 Jun;29(3):140-152. doi: 10.1017/neu.2016.56. Epub 2016 Oct 24. Acta Neuropsychiatr. 2017. PMID: 27772534 Review. - Glial reduction in amygdala in major depressive disorder is due to oligodendrocytes.
Hamidi M, Drevets WC, Price JL. Hamidi M, et al. Biol Psychiatry. 2004 Mar 15;55(6):563-9. doi: 10.1016/j.biopsych.2003.11.006. Biol Psychiatry. 2004. PMID: 15013824 - Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder.
Cotter D, Mackay D, Chana G, Beasley C, Landau S, Everall IP. Cotter D, et al. Cereb Cortex. 2002 Apr;12(4):386-94. doi: 10.1093/cercor/12.4.386. Cereb Cortex. 2002. PMID: 11884354 - Altered glial plasticity in animal models for mood disorders.
Czéh B, Fuchs E, Flügge G. Czéh B, et al. Curr Drug Targets. 2013 Oct;14(11):1249-61. doi: 10.2174/1389450111314110005. Curr Drug Targets. 2013. PMID: 23597041 Review.
Cited by
- Inflammation, Glutamate, and Glia: A Trio of Trouble in Mood Disorders.
Haroon E, Miller AH, Sanacora G. Haroon E, et al. Neuropsychopharmacology. 2017 Jan;42(1):193-215. doi: 10.1038/npp.2016.199. Epub 2016 Sep 15. Neuropsychopharmacology. 2017. PMID: 27629368 Free PMC article. Review. - Brain imaging in fibromyalgia.
Jorge LL, Amaro E Jr. Jorge LL, et al. Curr Pain Headache Rep. 2012 Oct;16(5):388-98. doi: 10.1007/s11916-012-0284-9. Curr Pain Headache Rep. 2012. PMID: 22717698 Review. - Increasing Adult Hippocampal Neurogenesis Promotes Resilience in a Mouse Model of Depression.
Planchez B, Lagunas N, Le Guisquet AM, Legrand M, Surget A, Hen R, Belzung C. Planchez B, et al. Cells. 2021 Apr 21;10(5):972. doi: 10.3390/cells10050972. Cells. 2021. PMID: 33919292 Free PMC article. - Circulating Plasma Micro RNAs in Patients with Major Depressive Disorder Treated with Antidepressants: A Pilot Study.
Enatescu VR, Papava I, Enatescu I, Antonescu M, Anghel A, Seclaman E, Sirbu IO, Marian C. Enatescu VR, et al. Psychiatry Investig. 2016 Sep;13(5):549-557. doi: 10.4306/pi.2016.13.5.549. Epub 2016 Sep 30. Psychiatry Investig. 2016. PMID: 27757134 Free PMC article.
References
- Kettenmann H, Ransom BR. In: Neuroglia. 2nd ed. Kettenmann H, Ransom BR, editors. Oxford University Press; New York: 2005. p. 1.
- Volterra A, Meldolesi J. Nat. Rev. Neurosci. 2005;6:626. - PubMed
- Seifert G, Schilling K, Steinhauser C. Nat. Rev. Neurosci. 2006;7:194. - PubMed
- Rajkowska G. The Neuroscientist. 2003;9:273. - PubMed
- Kandel ER. In: Principles of neuronal science. 4th ed. Kandel ER, Schwartz JH, Jessell TM, editors. McGraw-Hill; 2000.
Publication types
MeSH terms
Grants and funding
- P50 MH060451/MH/NIMH NIH HHS/United States
- MH60451/MH/NIMH NIH HHS/United States
- R01 MH061578/MH/NIMH NIH HHS/United States
- P20 RR017701-049007/RR/NCRR NIH HHS/United States
- MH67996/MH/NIMH NIH HHS/United States
- R01 MH063187/MH/NIMH NIH HHS/United States
- R01 MH063187-04/MH/NIMH NIH HHS/United States
- P20 RR017701/RR/NCRR NIH HHS/United States
- R01 MH061578-03/MH/NIMH NIH HHS/United States
- RR17701/RR/NCRR NIH HHS/United States
- MH61578/MH/NIMH NIH HHS/United States
- R01 MH067996-04/MH/NIMH NIH HHS/United States
- MH63187/MH/NIMH NIH HHS/United States
- P50 MH060451-03/MH/NIMH NIH HHS/United States
- R01 MH067996/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous