Bmp2 is critical for the murine uterine decidual response - PubMed (original) (raw)
Bmp2 is critical for the murine uterine decidual response
Kevin Y Lee et al. Mol Cell Biol. 2007 Aug.
Abstract
The process of implantation, necessary for all viviparous birth, consists of tightly regulated events, including apposition of the blastocyst, attachment to the uterine lumen, and differentiation of the uterine stroma. In rodents and primates the uterine stroma undergoes a process called decidualization. Decidualization, the process by which the uterine endometrial stroma proliferates and differentiates into large epithelioid decidual cells, is critical to the establishment of fetal-maternal communication and the progression of implantation. The role of bone morphogenetic protein 2 (Bmp2) in regulating the transformation of the uterine stroma during embryo implantation in the mouse was investigated by the conditional ablation of Bmp2 in the uterus using the (PR-cre) mouse. Bmp2 gene ablation was confirmed by real-time PCR analysis in the PR-cre; Bmp2fl/fl (termed Bmp2d/d) uterus. While littermate controls average 0.9 litter of 6.2+/-0.7 pups per month, Bmp2d/d females are completely infertile. Analysis of the infertility indicates that whereas embryo attachment is normal in the Bmp2d/d as in control mice, the uterine stroma is incapable of undergoing the decidual reaction to support further embryonic development. Recombinant human BMP2 can partially rescue the decidual response, suggesting that the observed phenotypes are not due to a developmental consequence of Bmp2 ablation. Microarray analysis demonstrates that ablation of Bmp2 leads to specific gene changes, including disruption of the Wnt signaling pathway, Progesterone receptor (PR) signaling, and the induction of prostaglandin synthase 2 (Ptgs2). Taken together, these data demonstrate that Bmp2 is a critical regulator of gene expression and function in the murine uterus.
Figures
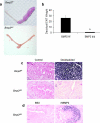
FIG. 1.
Decidualization in Bmp2 f/f and Bmp2 d/d mice. (a) Gross morphology of the decidual response in Bmp2 f/f and Bmp2 d/d uteri 5 days after the decidual stimulus. (b) Ratio of the decidual horn to the control horn weight of Bmp2 f/f and Bmp2 d/d uteri 5 days after the decidual stimulus. *, P = 3.8 e-4 from Bmp2 f/f mice. (c) Differentiation as measured by alkaline phosphatase staining in the Bmp2 f/f and_Bmp2_ d/d uterus 48 h after the decidual trauma. (d) Differentiation as measured by alkaline phosphatase staining in the Bmp2 d/d uterus treated with 10% BSA or 167 ng of human recombinant Bmp2/μl in 10% BSA 48 h after the decidual trauma. Original magnification, ×10.
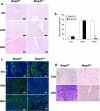
FIG. 2.
Bmp2 ablation affects differentiation and proliferation but not vascularization. (a) Proliferation as measured by immunohistochemical staining of phosphorylated histone H3 in Bmp2 f/f and Bmp2 d/d uteri prior to and 24 and 48 h after the decidual trauma. Slides are counterstained with nuclear fast red. (b) Quantification of phosphorylated histone H3-positive cells. The data are shown as means ± the standard error of the mean (SEM). Sections from three uteri of each genotype were counted at all time points. *, P = 8.36 e-5 from Bmp2 f/f at 48 h. (c) Vascularization as measured by CD31 immunofluorescence in Bmp2 f/f and Bmp2 d/d uteri prior to and 24 and 48 h after the decidual trauma. Slides are counterstained with DAPI. (d) Differentiation as measured by alkaline phosphatase staining in Bmp2 f/f and Bmp2 d/d uteri 24 and 48 h after the decidual trauma. Slides are counterstained with nuclear fast red. Original magnification, ×20.
FIG. 3.
Bmp2 ablation deregulates Wnt signaling. Real-time PCR in Wnt4 (a), Wnt6 (b), Sfrp4 (c), and Dact1 in Bmp2 f/f and Bmp2 d/d uteri. The data shown as means ± the SEM. *, P < 0.01 from Bmp2 f/f at the corresponding time point; **, P < 0.05 from Bmp2 f/f at the corresponding time point.
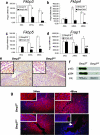
FIG. 4.
Bmp2 ablation deregulates the expression and action of FK-506-binding proteins. Real-time PCR in Fkbp3 (a), Fkbp4 (b), Fkbp5 (c), and Frap1 (d) in Bmp2 f/f and Bmp2 d/d uteri. The data shown as means ± the SEM. *, P < 0.01 from Bmp2 f/f at the corresponding time point; **, P < 0.05 from Bmp2 f/f at the corresponding time point. (e) PR immunohistochemistry in Bmp2 f/f and Bmp2 d/d uteri 24 h after the decidual trauma. (f) Western blot for phosphorylated and total PR 24 h after the decidual trauma. (g) Expression of Frap1 by immunofluorescence. The arrow indicates retained epithelial expression. Original magnification, ×20. High-power insets are at ×40 magnification.
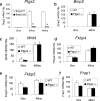
FIG. 5.
Bmp2 acts upstream of Ptgs2. (a) Real-time PCR in Ptgs2 in Bmp2 f/f and Bmp2 d/d uteri. Real-time PCR of Bmp2 (b), Wnt4 (c), Fkbp4 (d), Fkbp5 (e), and Frap1 (f), in _Ptgs2_−/− and littermate control uteri. The data shown as means ± the SEM. *, P < 0.01 from Bmp2 f/f at the corresponding time point; *, P < 0.05 from Bmp2 f/f at the corresponding time point.
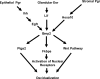
FIG. 6.
Model depicting the relationships of genes during implantation.
Similar articles
- Bone morphogenetic protein 2 functions via a conserved signaling pathway involving Wnt4 to regulate uterine decidualization in the mouse and the human.
Li Q, Kannan A, Wang W, Demayo FJ, Taylor RN, Bagchi MK, Bagchi IC. Li Q, et al. J Biol Chem. 2007 Oct 26;282(43):31725-32. doi: 10.1074/jbc.M704723200. Epub 2007 Aug 21. J Biol Chem. 2007. PMID: 17711857 - Cellular and molecular responses of the uterus to embryo implantation can be elicited by locally applied growth factors.
Paria BC, Ma W, Tan J, Raja S, Das SK, Dey SK, Hogan BL. Paria BC, et al. Proc Natl Acad Sci U S A. 2001 Jan 30;98(3):1047-52. doi: 10.1073/pnas.98.3.1047. Proc Natl Acad Sci U S A. 2001. PMID: 11158592 Free PMC article. - Activin-like kinase 2 functions in peri-implantation uterine signaling in mice and humans.
Clementi C, Tripurani SK, Large MJ, Edson MA, Creighton CJ, Hawkins SM, Kovanci E, Kaartinen V, Lydon JP, Pangas SA, DeMayo FJ, Matzuk MM. Clementi C, et al. PLoS Genet. 2013 Nov;9(11):e1003863. doi: 10.1371/journal.pgen.1003863. Epub 2013 Nov 14. PLoS Genet. 2013. PMID: 24244176 Free PMC article. - Implantation in the baboon: endometrial responses.
Fazleabas AT, Kim JJ, Srinivasan S, Donnelly KM, Brudney A, Jaffe RC. Fazleabas AT, et al. Semin Reprod Endocrinol. 1999;17(3):257-65. doi: 10.1055/s-2007-1016233. Semin Reprod Endocrinol. 1999. PMID: 10797944 Review. - Cell cycle regulatory control for uterine stromal cell decidualization in implantation.
Das SK. Das SK. Reproduction. 2009 Jun;137(6):889-99. doi: 10.1530/REP-08-0539. Epub 2009 Mar 23. Reproduction. 2009. PMID: 19307426 Review.
Cited by
- Bmp2 deletion causes an amelogenesis imperfecta phenotype via regulating enamel gene expression.
Guo F, Feng J, Wang F, Li W, Gao Q, Chen Z, Shoff L, Donly KJ, Gluhak-Heinrich J, Chun YH, Harris SE, MacDougall M, Chen S. Guo F, et al. J Cell Physiol. 2015 Aug;230(8):1871-82. doi: 10.1002/jcp.24915. J Cell Physiol. 2015. PMID: 25545831 Free PMC article. - Distinct Murine Pancreatic Transcriptomic Signatures during Chronic Pancreatitis Recovery.
Zhang Y, Yang B, Davis JM, Drake MM, Younes M, Shen Q, Zhao Z, Cao Y, Ko TC. Zhang Y, et al. Mediators Inflamm. 2021 May 15;2021:5595464. doi: 10.1155/2021/5595464. eCollection 2021. Mediators Inflamm. 2021. PMID: 34104113 Free PMC article. - Uterine deletion of Gp130 or Stat3 shows implantation failure with increased estrogenic responses.
Sun X, Bartos A, Whitsett JA, Dey SK. Sun X, et al. Mol Endocrinol. 2013 Sep;27(9):1492-501. doi: 10.1210/me.2013-1086. Epub 2013 Jul 24. Mol Endocrinol. 2013. PMID: 23885093 Free PMC article. - ID3 mediates BMP2-induced downregulation of ICAM1 expression in human endometiral stromal cells and decidual cells.
Luo J, Wang Y, Chang HM, Zhu H, Yang J, Leung PCK. Luo J, et al. Front Cell Dev Biol. 2023 Feb 24;11:1090593. doi: 10.3389/fcell.2023.1090593. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36910152 Free PMC article. - Wnt4, Wnt6 and β-catenin expression in human placental tissue - is there a link with first trimester miscarriage? Results from a pilot study.
Chronopoulou E, Koika V, Tsiveriotis K, Stefanidis K, Kalogeropoulos S, Georgopoulos N, Adonakis G, Kaponis A. Chronopoulou E, et al. Reprod Biol Endocrinol. 2022 Mar 17;20(1):51. doi: 10.1186/s12958-022-00923-4. Reprod Biol Endocrinol. 2022. PMID: 35300692 Free PMC article.
References
- Barent, R. L., S. C. Nair, D. C. Carr, Y. Ruan, R. A. Rimerman, J. Fulton, Y. Zhang, and D. F. Smith. 1998. Analysis of FKBP51/FKBP52 chimeras and mutants for Hsp90 binding and association with progesterone receptor complexes. Mol. Endocrinol. 12:342-354. - PubMed
- Brenner, R. M., O. D. Slayden, W. H. Rodgers, H. O. Critchley, R. Carroll, X. J. Nie, and K. Mah. 2003. Immunocytochemical assessment of mitotic activity with an antibody to phosphorylated histone H3 in the macaque and human endometrium. Hum. Reprod. 18:1185-1193. - PubMed
- Brott, B. K., and S. Y. Sokol. 2005. Frodo proteins: modulators of Wnt signaling in vertebrate development. Differentiation 73:323-329. - PubMed
- Canalis, E., A. N. Economides, and E. Gazzerro. 2003. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocrinol. Rev. 24:218-235. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK055636/DK/NIDDK NIH HHS/United States
- R01 DE012324-11/DE/NIDCR NIH HHS/United States
- 5 T32 HD07165/HD/NICHD NIH HHS/United States
- T32 HD007165/HD/NICHD NIH HHS/United States
- U01 HD042311/HD/NICHD NIH HHS/United States
- R01 CA077530/CA/NCI NIH HHS/United States
- R01-CA77530/CA/NCI NIH HHS/United States
- R01-DK55636/DK/NIDDK NIH HHS/United States
- U01HD042311/HD/NICHD NIH HHS/United States
- R01 DE012324/DE/NIDCR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials