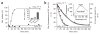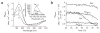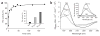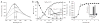The redox-switch domain of Hsp33 functions as dual stress sensor - PubMed (original) (raw)
Comparative Study
The redox-switch domain of Hsp33 functions as dual stress sensor
Marianne Ilbert et al. Nat Struct Mol Biol. 2007 Jun.
Abstract
The redox-regulated chaperone Hsp33 is specifically activated upon exposure of cells to peroxide stress at elevated temperatures. Here we show that Hsp33 harbors two interdependent stress-sensing regions located in the C-terminal redox-switch domain of Hsp33: a zinc center sensing peroxide stress conditions and an adjacent linker region responding to unfolding conditions. Neither of these sensors works sufficiently in the absence of the other, making the simultaneous presence of both stress conditions a necessary requirement for Hsp33's full activation. Upon activation, Hsp33's redox-switch domain adopts a natively unfolded conformation, thereby exposing hydrophobic surfaces in its N-terminal substrate-binding domain. The specific activation of Hsp33 by the oxidative unfolding of its redox-switch domain makes this chaperone optimally suited to quickly respond to oxidative stress conditions that lead to protein unfolding.
Conflict of interest statement
COMPETING INTERESTS STATEMENT
The authors declare no competing financial interests.
Figures
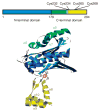
Figure 1
Domain structure of Hsp33. Linear representation (top) is color coded similarly to structural model of Hsp33 monomer (bottom), which is based on the crystal structure of the reduced Bacillus subtilis Hsp33 dimer (PDB 1VZY).
Figure 2
In vitro activation of Hsp33 requires oxidative stress at elevated temperatures. (a) Activation of Hsp33 in vitro requires simultaneous presence of oxidants and elevated temperature. Hsp33red was incubated with H2O2 at either 30 °C (●) or 43 °C (○). At various time points, chaperone activity in aliquots was determined by measuring aggregation of chemically denatured citrate synthase at 30 °C. Light-scattering signal 4 min after addition of citrate synthase is plotted against incubation time; signal in the absence of Hsp33 was defined as 0% activity, and signal with fully active Hsp33DCCSSS mutant protein (which forms intermolecular dimers during its purification) as 100%. Inset, activity of Hsp33red, Hsp33ox30°C and Hsp33ox43°C with chemically denatured citrate synthase at 43 °C. (b) Temperature dependence of Hsp33’s oxidative zinc release. Reduced, zinc-reconstituted Hsp33(W212F Y267W) was incubated with H2O2 at 30 °C (triangles) or 43 °C (circles) and tryptophan fluorescence was monitored (open symbols, left axis; see Methods). Simultaneously, zinc-binding affinities of Hsp33(W212F Y267W) aliquots were analyzed using the PAR/PCMB assay (filled symbols, right axis). 100%, values for Hsp33(W212F Y267W)red; 0%, Hsp33(W212F Y267W)ox43°C (oxidized for 3 h). Inset, Hsp33(W212F Y267W) shows zinc-dependent tryptophan fluorescence. Fluorescence spectrum of zinc-reconstituted Hsp33(W212F Y267W) or zinc-depleted apo-Hsp33 was monitored under reducing conditions. AU, arbitrary units.
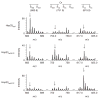
Figure 3
Thiol status of Hsp33’s tryptic peptide 232-CTCSR-236 (calculated m/z = 569.7), containing the first redox-active cysteine pair. Wild-type Hsp33red, Hsp33ox30°C or Hsp33ox43°C (oxidized for 1 h) was incubated with IAM, then reduced by DTT and treated with NEM. Mass spectra of the differentially labeled tryptic peptides were obtained by MALDI-MS. m/z value of 683.8 represents the fully reduced (IAM-labeled) peptide; 819.9, fully oxidized (NEM-labeled) peptide; 751.9, partially oxidized intermediate harboring one NEM and one IAM label. The identities of the peaks were confirmed by MS/MS analysis. Arrows mark expected theoretical mass of the modified peptide.
Figure 4
Activation of Hsp33 is accompanied by major conformational rearrangements. (a) Far-UV CD spectra of Hsp33red (1, solid line), Hsp33ox30°C (2, dotted line), Hsp33Cys− (3, dotted and dashed line) and Hsp33ox43°C (4, dashed line) were recorded at 30 °C, whereas Hsp33Cys− (5, double dotted and dashed line) was recorded at 43 °C. Inset, bis-ANS fluorescence to monitor surface hydrophobicity in reduced and oxidized Hsp33. 10 μM bis-ANS was incubated with 3 μM Hsp33red, Hsp33ox30°C or Hsp33ox43°C. Emission spectra were recorded using _λ_ex of 370 nm at 30 °C. (b) Oxidative zinc release destabilizes additional regions in Hsp33. Analysis of temperature-induced conformational changes by monitoring the changes in molecular ellipticity at 197 nm. Hsp33red, Hsp33ox30°C, Hsp33Cys− or Hsp33ox43°C was heated to 50 °C (solid line), then cooled to 20 °C (dotted line).
Figure 5
C-terminal truncation mutant Hsp331–235 functions as a redox-regulated chaperone. (a) Activation kinetics of Hsp331–235 upon oxidation at 43 °C. 50 μM Hsp331–235(red) was incubated with H2O2 at 43 °C and chaperone activity was determined as in Figure 2a. Inset, influence of Hsp331–235(red) (in 1 mM DTT), Hsp331–235(ox30°C) or Hsp331–235(ox43°C) on aggregation of chemically unfolded citrate synthase measured at 43 °C. (b) Activation of Hsp331–235 is accompanied by major conformational changes, shown by far-UV CD spectra of Hsp331–235(red), Hsp331–235(ox30°C) or Hsp331–235(ox43°C) at 30 °C. Inset, bis-ANS binding to monitor hydrophobic surfaces in Hsp331–235(red), Hsp331–235(ox30°C) and Hsp331–235(ox43°C) as in Figure 4a. AU, arbitrary units.
Figure 6
Unfolding of the linker region is crucial for Hsp33’s activation. (a) Fluorescence spectra of Hsp33(F187W W212F)red, Hsp33(F187W W212F)ox30°C and Hsp33(F187W W212F)ox43°C. AU, arbitrary units. (b) Correlation between zinc release and conformational changes in Hsp33’s linker region upon oxidation of Hsp33 at 30 and 43 °C. Hsp33(F187W W212F)red was incubated with H2O2 at 43 °C (larger chart) or 30 °C (inset). Emission maxima of fluorescence spectra are plotted against incubation time (○, right axis). At indicated times, zinc release in aliquots was also measured using the PAR-PCMB assay (●, left axis). (c) Stability of Hsp33(F187W W212F) incubated in Gdn-HCl for 24 h (supplemented with 5 mM DTT). Tryptophan fluorescence was measured; wavelength of the emission maximum (_λ_max) is plotted against Gdn-HCl concentration. Arrow indicates _λ_max of active Hsp33(F187W W212F) after 3 h of incubation in 2 mM H2O2 at 43 °C. Inset, oxidative activation of Hsp33 in 1.5 M Gdn-HCl at 30 °C. Hsp33red was incubated with H2O2 at 30 °C in the absence (ox30°C) or presence of 1.5 M Gdn-HCl (ox30°C/Gdn) or at 43 °C (ox43°C). As control, Hsp33red was incubated in 1.5 M Gdn-HCl in the absence of oxidizing reagents (red/Gdn). The activity of Hsp33 was determined as in Figure 2a.
Figure 7
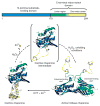
Schematic model of Hsp33’s activation process. Upon oxidation of Hsp33 at 30 °C, zinc is released and the zinc center (α-helices 8–10) unfolds. This unfolding is apparently not sufficient to activate the chaperone function, but generates an oxidation intermediate of Hsp33 (shown in brackets), which is presumably only very transiently populated during oxidation at 43 °C. Upon exposure to oxidizing and unfolding conditions—for example, H2O2 at elevated temperature—the complete C terminus (α-helices 5–10) converts to a natively unfolded protein. These extensive conformational rearrangements lead to the exposure of hydrophobic surfaces, presumably on the N-terminal substrate-binding domain of Hsp33, and allow Hsp33 to dimerize.
Similar articles
- Unfolding of metastable linker region is at the core of Hsp33 activation as a redox-regulated chaperone.
Cremers CM, Reichmann D, Hausmann J, Ilbert M, Jakob U. Cremers CM, et al. J Biol Chem. 2010 Apr 9;285(15):11243-51. doi: 10.1074/jbc.M109.084350. Epub 2010 Feb 5. J Biol Chem. 2010. PMID: 20139072 Free PMC article. - Activation of the redox-regulated molecular chaperone Hsp33--a two-step mechanism.
Graumann J, Lilie H, Tang X, Tucker KA, Hoffmann JH, Vijayalakshmi J, Saper M, Bardwell JC, Jakob U. Graumann J, et al. Structure. 2001 May 9;9(5):377-87. doi: 10.1016/s0969-2126(01)00599-8. Structure. 2001. PMID: 11377198 - A Role of Metastable Regions and Their Connectivity in the Inactivation of a Redox-Regulated Chaperone and Its Inter-Chaperone Crosstalk.
Rimon O, Suss O, Goldenberg M, Fassler R, Yogev O, Amartely H, Propper G, Friedler A, Reichmann D. Rimon O, et al. Antioxid Redox Signal. 2017 Nov 20;27(15):1252-1267. doi: 10.1089/ars.2016.6900. Epub 2017 Apr 10. Antioxid Redox Signal. 2017. PMID: 28394178 - Redox-regulated molecular chaperones.
Graf PC, Jakob U. Graf PC, et al. Cell Mol Life Sci. 2002 Oct;59(10):1624-31. doi: 10.1007/pl00012489. Cell Mol Life Sci. 2002. PMID: 12475172 Free PMC article. Review. - Oxidative stress: Protein folding with a novel redox switch.
Ruddock LW, Klappa P. Ruddock LW, et al. Curr Biol. 1999 Jun 3;9(11):R400-2. doi: 10.1016/s0960-9822(99)80253-x. Curr Biol. 1999. PMID: 10359689 Review.
Cited by
- Unfolding of metastable linker region is at the core of Hsp33 activation as a redox-regulated chaperone.
Cremers CM, Reichmann D, Hausmann J, Ilbert M, Jakob U. Cremers CM, et al. J Biol Chem. 2010 Apr 9;285(15):11243-51. doi: 10.1074/jbc.M109.084350. Epub 2010 Feb 5. J Biol Chem. 2010. PMID: 20139072 Free PMC article. - Are zinc-finger domains of protein kinase C dynamic structures that unfold by lipid or redox activation?
Zhao F, Ilbert M, Varadan R, Cremers CM, Hoyos B, Acin-Perez R, Vinogradov V, Cowburn D, Jakob U, Hammerling U. Zhao F, et al. Antioxid Redox Signal. 2011 Mar 1;14(5):757-66. doi: 10.1089/ars.2010.3773. Epub 2011 Jan 4. Antioxid Redox Signal. 2011. PMID: 21067413 Free PMC article. - Bleach activates a redox-regulated chaperone by oxidative protein unfolding.
Winter J, Ilbert M, Graf PC, Ozcelik D, Jakob U. Winter J, et al. Cell. 2008 Nov 14;135(4):691-701. doi: 10.1016/j.cell.2008.09.024. Cell. 2008. PMID: 19013278 Free PMC article. - From guide to guard-activation mechanism of the stress-sensing chaperone Get3.
Ulrich K, Farkas Á, Chan O, Katamanin O, Schwappach B, Jakob U. Ulrich K, et al. Mol Cell. 2022 Sep 1;82(17):3226-3238.e7. doi: 10.1016/j.molcel.2022.06.015. Epub 2022 Jul 14. Mol Cell. 2022. PMID: 35839781 Free PMC article. - The molecular chaperone Hsp33 is activated by atmospheric-pressure plasma protecting proteins from aggregation.
Krewing M, Stepanek JJ, Cremers C, Lackmann JW, Schubert B, Müller A, Awakowicz P, Leichert LIO, Jakob U, Bandow JE. Krewing M, et al. J R Soc Interface. 2019 Jun 28;16(155):20180966. doi: 10.1098/rsif.2018.0966. Epub 2019 Jun 19. J R Soc Interface. 2019. PMID: 31213177 Free PMC article.
References
- Imlay JA. Pathways of oxidative damage. Annu Rev Microbiol. 2003;57:395–418. - PubMed
- Holmgren A. Antioxidant function of thioredoxin and glutaredoxin systems. Antioxid Redox Signal. 2000;2:811–820. - PubMed
- Sies H. Oxidative stress: from basic research to clinical application. Am J Med. 1991;91:31S–38S. - PubMed
- Paget MS, Buttner MJ. Thiol-based regulatory switches. Annu Rev Genet. 2003;37:91–121. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases