Engineering complex tissues - PubMed (original) (raw)
Review
. 2006 Dec;12(12):3307-39.
doi: 10.1089/ten.2006.12.3307.
Susan W Herring, Pannee Ochareon, Jennifer Elisseeff, Helen H Lu, Rita Kandel, Frederick J Schoen, Mehmet Toner, David Mooney, Anthony Atala, Mark E Van Dyke, David Kaplan, Gordana Vunjak-Novakovic
Affiliations
- PMID: 17518671
- PMCID: PMC2821210
- DOI: 10.1089/ten.2006.12.3307
Review
Engineering complex tissues
Antonios G Mikos et al. Tissue Eng. 2006 Dec.
Abstract
This article summarizes the views expressed at the third session of the workshop "Tissue Engineering--The Next Generation," which was devoted to the engineering of complex tissue structures. Antonios Mikos described the engineering of complex oral and craniofacial tissues as a "guided interplay" between biomaterial scaffolds, growth factors, and local cell populations toward the restoration of the original architecture and function of complex tissues. Susan Herring, reviewing osteogenesis and vasculogenesis, explained that the vascular arrangement precedes and dictates the architecture of the new bone, and proposed that engineering of osseous tissues might benefit from preconstruction of an appropriate vasculature. Jennifer Elisseeff explored the formation of complex tissue structures based on the example of stratified cartilage engineered using stem cells and hydrogels. Helen Lu discussed engineering of tissue interfaces, a problem critical for biological fixation of tendons and ligaments, and the development of a new generation of fixation devices. Rita Kandel discussed the challenges related to the re-creation of the cartilage-bone interface, in the context of tissue engineered joint repair. Frederick Schoen emphasized, in the context of heart valve engineering, the need for including the requirements derived from "adult biology" of tissue remodeling and establishing reliable early predictors of success or failure of tissue engineered implants. Mehmet Toner presented a review of biopreservation techniques and stressed that a new breakthrough in this field may be necessary to meet all the needs of tissue engineering. David Mooney described systems providing temporal and spatial regulation of growth factor availability, which may find utility in virtually all tissue engineering and regeneration applications, including directed in vitro and in vivo vascularization of tissues. Anthony Atala offered a clinician's perspective for functional tissue regeneration, and discussed new biomaterials that can be used to develop new regenerative technologies.
Figures
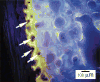
FIG. 1
Lateral surface of the zygomatic bone, coronal section, epifluorescent illumination. Light color is the 3-hour calcein label in a continuous layer of newly mineralized matrix. Vascular fill (red) is seen in the periosteal vessels and in cross-sectioned vessels within the calcein-labeled layer (arrows). Color images available online at
.
FIG. 2
Lateral surface of the temporal bone, coronal section, epifluorescent illumination. The 3-hour calcein label is discontinuous and forms the tips of bony spicules. Vascular fill (red) shows that the radially arranged vessels of this layer are continuous with intraosseous vessels (arrows) and differ in orientation from the periosteal vessels. Color images available online at
.
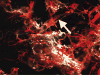
FIG. 3
Lateral portion of the zygomatic bone, parasagittal section. An intraosseous vessel near the surface can be seen encased in a bony tube (arrow). Color images available online at
.
FIG. 4
Cartilage has been engineered using a number of different cell types. Primary bovine chondrocytes (A), caprine MSCs (B), mouse EBs (C), and human EBs (D) demonstrate Safranin-O positive proteoglycan production in polyethylene glycol (PEG) gels with the exception of human EBs, which did not undergo chondrogenesis. Human embryonic stem (ES) cells were aggregated into EBs and either encapsulated directly (2A) or disaggregated and cultured for five passages (1) before encapsulation (2B). The hEBs that were encapsulated into PEG gels did not stain positive for Safranin-O (D). Cells derived from the hEBs (1) encapsulated in PEG gels also did not stain positive for Safranin-O when cultured in chondrogenic medium with TGF-β1 (E). Incorporation of the adhesion peptide sequence YRGDS into the PEG gels promoted homogenous differentiation and formation of cartilage-like tissue from hES-derived cells (F), confirming the unique requirements for human ES cell differentiation. Reproduced with permission from Annals of Biomedical Engineering. Color images available online at
.
FIG. 5
Cells labeled with colored dyes encapsulated in a bilayered hydrogel. Organized tissue can be created or interactions of coculture environments can be studied. Reproduced with permission from Annals of Biomedical Engineering. Color images available online at
.
FIG. 6
Anatomy and matrix organization of the ligament-to-bone insertion site. (A) The anterior cruciate ligament (ACL) connects to the femur and tibia through two insertion sites (posterior view). (B) The multitissue organization of the tibial insertion, transiting from the ACL to fibrocartilage (FC) region, and then to the bone region (modified Goldner’s Masson Trichrome, bar = 500 μm). (C) The fibrocartilage interface is further divided into the nonmineralized fibrocartilage (NFC) and mineralized fibrocartilage (MFC) zones (von Kossa, bar = 200 μm). Color images available online at
.
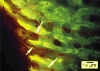
FIG. 7
Coculture models to evaluate interaction of interface-relevant cells. (A-i) In vitro coculture model of fibroblasts (Fb) and osteoblasts (Ob) permit heterotypic and homotypic cell-cell interactions. (A-ii) Fibroblast (CFDA-SE, green) and osteoblast (CM-DiI, orange-red) distribution at day 7, bar = 100 μm. (B) In vitro coculture model of chondrocytes (Ch) and osteoblasts (Ob), established by forming an osteoblast monolayer on top of the chondrocyte micromass. Glycosaminoglycan distribution was restricted to the chondrocytes micromass during coculture (day 10, Alcian blue, bar = 100 μm). Color images available online at
.
FIG. 8
Structure-function relationship at the ligament-to-bone insertion. (A-i) Elastographic analysis of the ACL-to-bone insertion (TI) under applied uniaxial tension. Displacement map calculated from ultrasound radio frequency data (Increase in magnitude in mm: blue to red, bar = 5 mm). A region-dependent decrease in displacement is a result of increasing tissue stiffness from the ligament to fibrocartilage region and to the bone region. (A-ii) Microcompression testing of the ACL-to-bone insertion also revealed region-specific increase in tissue stiffness from the nonmineralized (NFC) to mineralized fibrocartilage (MFC) and to bone. In the displacement curve, slope of the curve in each region represents the strain, with a less steeper slope for MFC, indicating decreased strain compared to the NFC zone. (B) The increase in tissue stiffness across the interface may be related to the higher calcium phosphate distribution from the NFC to MFC, and to bone (i von Kossa and ii Elemental analysis of the MFC revealed presence of Ca and P at the insertion104). Color images available online at
.
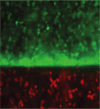
FIG. 9
Biomimetic multiphasic scaffold for interface tissue engineering: Design, in vitro and in vivo testing. (A) Triphasic scaffold modeled after the three regions of the interface: Phase A for soft tissue, Phase B for the fibrocartilage region, and Phase C for bone. Phase A consists of knitted degradable polymer mesh, Phase B of sintered degradable polymer microspheres, and Phase C of osteointegrative polymer-ceramic composite microspheres. (B) In vitro coculture of fibroblasts and osteoblasts on the triphasic scaffold resulted in phase-specific cell distribution and controlled matrix heterogeneity. Fibroblasts (Calcein AM, green) were localized in Phase A and osteoblasts (CM-DiI, red) were found in Phase C at day 1 (i) and day 28 (ii). Both osteoblasts and fibroblasts migrated into Phase B by day 28. (C) In vivo evalution of the triphasic scaffold cocutlured with fibroblasts and osteoblasts revealed abundant tissue infiltration and matrix production at 4 weeks postimplantation. (modified Goldner’s MassonTrichrome, bar = 200 μm). Color images available online at
.
FIG. 10
(A) Line drawing of biphasic construct. (B) Histological appearance of biphasic construct at 8 weeks of culture. The cartilage is integrated with the upper aspect of substrate and has a nonmineralized zone and a mineralized zone (arrowhead) adjacent to the substrate (von Kossa and toluidine blue, ×50 original magnification). Color images available online at
.
FIG. 11
Paradigm for translating research in heart valve tissue engineering from the laboratory to the clinic. Biomarkers for cell and tissue characterization in conjunction with structural, chemical, and molecular information obtained via in vitro and in vivo models are necessary for understanding key biological processes in tissue engineering and regenerative medicine. These concepts and data can be used to predict and measure patient success and failure. Data from clinical experience further informs the development of appropriate biomarkers, which may result in reassessment of the appropriate characterization parameters. Reproduced and modified with permission from Mendelson et al. Color images available online at
.
FIG. 12
Long-term storage of cells is critical for the successful applications of tissue engineered products. Cell and tissue banks are needed at various steps in the development of tissue engineered products. Reprinted with permission from Acker, J.P., Chen, T., Fowler, A., and Toner, M. Engineering dessication tolerance in mammalian cells: tools and techniques. In: Fuller, B.J., Lane, N., and Benson, E.E., eds. Life in the Frozen State. Boca Raton, FL: CRC Press, 2004. Color images available online at
.
Similar articles
- Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review. - Review of Machine Learning Techniques in Soft Tissue Biomechanics and Biomaterials.
Donmazov S, Saruhan EN, Pekkan K, Piskin S. Donmazov S, et al. Cardiovasc Eng Technol. 2024 Oct;15(5):522-549. doi: 10.1007/s13239-024-00737-y. Epub 2024 Jul 2. Cardiovasc Eng Technol. 2024. PMID: 38956008 Review. - Defining the optimum strategy for identifying adults and children with coeliac disease: systematic review and economic modelling.
Elwenspoek MM, Thom H, Sheppard AL, Keeney E, O'Donnell R, Jackson J, Roadevin C, Dawson S, Lane D, Stubbs J, Everitt H, Watson JC, Hay AD, Gillett P, Robins G, Jones HE, Mallett S, Whiting PF. Elwenspoek MM, et al. Health Technol Assess. 2022 Oct;26(44):1-310. doi: 10.3310/ZUCE8371. Health Technol Assess. 2022. PMID: 36321689 Free PMC article. - Qualitative evidence synthesis informing our understanding of people's perceptions and experiences of targeted digital communication.
Ryan R, Hill S. Ryan R, et al. Cochrane Database Syst Rev. 2019 Oct 23;10(10):ED000141. doi: 10.1002/14651858.ED000141. Cochrane Database Syst Rev. 2019. PMID: 31643081 Free PMC article. - Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.
Lee CC, Chen CW, Yen HK, Lin YP, Lai CY, Wang JL, Groot OQ, Janssen SJ, Schwab JH, Hsu FM, Lin WH. Lee CC, et al. Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
Cited by
- In Vitro Multitissue Interface Model Supports Rapid Vasculogenesis and Mechanistic Study of Vascularization across Tissue Compartments.
Buno KP, Chen X, Weibel JA, Thiede SN, Garimella SV, Yoder MC, Voytik-Harbin SL. Buno KP, et al. ACS Appl Mater Interfaces. 2016 Aug 31;8(34):21848-60. doi: 10.1021/acsami.6b01194. Epub 2016 May 2. ACS Appl Mater Interfaces. 2016. PMID: 27136321 Free PMC article. - Infiltration of chitin by protein coacervates defines the squid beak mechanical gradient.
Tan Y, Hoon S, Guerette PA, Wei W, Ghadban A, Hao C, Miserez A, Waite JH. Tan Y, et al. Nat Chem Biol. 2015 Jul;11(7):488-95. doi: 10.1038/nchembio.1833. Epub 2015 Jun 8. Nat Chem Biol. 2015. PMID: 26053298 - Nanopatterned acellular valve conduits drive the commitment of blood-derived multipotent cells.
Di Liddo R, Aguiari P, Barbon S, Bertalot T, Mandoli A, Tasso A, Schrenk S, Iop L, Gandaglia A, Parnigotto PP, Conconi MT, Gerosa G. Di Liddo R, et al. Int J Nanomedicine. 2016 Oct 12;11:5041-5055. doi: 10.2147/IJN.S115999. eCollection 2016. Int J Nanomedicine. 2016. PMID: 27789941 Free PMC article. - Two-photon polymerization for 3D biomedical scaffolds: Overview and updates.
Jing X, Fu H, Yu B, Sun M, Wang L. Jing X, et al. Front Bioeng Biotechnol. 2022 Aug 22;10:994355. doi: 10.3389/fbioe.2022.994355. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36072288 Free PMC article. Review. - Solid Organ Bioprinting: Strategies to Achieve Organ Function.
Jorgensen AM, Yoo JJ, Atala A. Jorgensen AM, et al. Chem Rev. 2020 Oct 14;120(19):11093-11127. doi: 10.1021/acs.chemrev.0c00145. Epub 2020 Sep 4. Chem Rev. 2020. PMID: 32885956 Free PMC article. Review.
References
- Yoshikawa H, Myoui A. Bone tissue engineering with porous hydroxyapatite ceramics. J Artif Organs. 2005;8:131. - PubMed
- Villanueva JE, Nimni ME. Promotion of calvarial cell osteogenesis by endothelial cells. J Bone Miner Res. 1990;5:733. - PubMed
- Carvalho RS, Einhorn TA, Lehmann W, Edgar C, Al-Yamani A, Apazidis A, Pacicca DM, Clemens TL, Gerstenfeld LC. The role of angiogenesis in a murine tibial model of distraction osteogenesis. J Artif Organs. 2004;34:849. - PubMed
- Krompecher S. Die Entwicklung der Knochenzellen und die Bildung der Knochengrundsubstanz bei der Knorpelig and bindegewebig vorgebildeten sowie der primären reinen Knochenbildung. Anat Anz. 1934;78:34.
Publication types
MeSH terms
Substances
Grants and funding
- AR052402/AR/NIAMS NIH HHS/United States
- R21 AR052402/AR/NIAMS NIH HHS/United States
- R01 DE15164/DE/NIDCR NIH HHS/United States
- P41 EB002520/EB/NIBIB NIH HHS/United States
- R01 DE008513/DE/NIDCR NIH HHS/United States
- R01 DE015164/DE/NIDCR NIH HHS/United States
- DE08513/DE/NIDCR NIH HHS/United States
- R01 DE008513-18/DE/NIDCR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous