Xenopus Bicaudal-C is required for the differentiation of the amphibian pronephros - PubMed (original) (raw)
Xenopus Bicaudal-C is required for the differentiation of the amphibian pronephros
Uyen Tran et al. Dev Biol. 2007.
Abstract
The RNA-binding molecule Bicaudal-C regulates embryonic development in Drosophila and Xenopus. Interestingly, mouse mutants of Bicaudal-C do not show early patterning defects, but instead develop polycystic kidney disease (PKD). To further investigate the molecular mechanism of Bicaudal-C in kidney development, we analyzed its function in the developing amphibian pronephros. Bicaudal-C mRNA was present in the epithelial structures of the Xenopus pronephros, the tubules and the duct, but not the glomus. Inhibition of the translation of endogenous Bicaudal-C with antisense morpholino oligomers (xBic-C-MO) led to a PKD-like phenotype in Xenopus. Embryos lacking Bicaudal-C developed generalized edemas and dilated pronephric tubules and ducts. This phenotype was caused by impaired differentiation of the pronephros. Molecular markers specifically expressed in the late distal tubule were absent in xBic-C-MO-injected embryos. Furthermore, Bicaudal-C was not required for primary cilia formation, an important organelle affected in PKD. These data support the idea that Bicaudal-C functions downstream or parallel of a cilia-regulated signaling pathway. This pathway is required for terminal differentiation of the late distal tubule of the Xenopus pronephros and regulates renal epithelial cell differentiation, which--when disrupted--results in PKD.
Figures
Figure 1. Pronephros Expression of xBic-C
(A-C) Whole mount in situ hybridization analysis of xBic-C at (A) stage 30 and (B) stage 38 showing expression in the pronephric tubules and duct. (C) Close up of B; arrows indicate the expression of Bicaudal-C in the three nephrostomes. (D) Schematic diagram of the amphibian pronephros. (E,F) Anterior and posterior transverse section of (B) showing xBic-C expression in the tubules and duct, respectively. (G) Double in situ hybridization with WT-1 (purple) and xBic-C (turquoise). Note that xBic-C is expressed only in the epithelial components of the pronephros, whereas, WT-1 is expressed specifically in the glomus. Insert in E-G are close-ups of the pronephric region highlighting the epithelium-specific expression of Bicaudal-C.
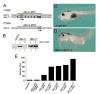
Figure 2. Inhibition of xBic-C Leads to Edema Formation
(A) Sequence of the two pseudoalleles of Xenopus Bicaudal-C. The positions of the two antisense morpholino oligomers are indicated. (B) In vitro transcription/translation of xBic-C in the presence or absence of xBic-C-MO1. Lane 1, pCSII; lane 2 and 3, _xBic-C-HA_-pCSII; lane 4 and 5, _xBic-C*-HA_-pCSII. (C,D) Morphology of uninjected and xBic-C-MO1+_2_-injected embryo displaying large edema. (E) Quantification of edema formation at stage 43 of uninjected control embryos and Xenopus embryos injected radially at the 2-4 cell stage with xBic-C-MO2-scrambled (1 pMol), xBic-C-MO1 (1 pMol and 2 pMol), xBic-C-MO2 (1 pMol and 2 pMol), and xBic-C-MO1+2 (1 pMol each morpholino oligomer).
Figure 3. Bicaudal-C is Not Required for Pronephros Formation
(A-D) Histological analysis of transverse sections from uninjected and xBic-C-MO1+_2_-injected embryos at stage 45 (A,B) and stage 38 (C,D). Note the edema and the enlarged pronephric tubules in embryos lacking xBic-C. (E,F) Whole mount in situ hybridization of β1-Na/K ATPase of uninjected and xBic-C-MO1+_2_-injected embryos. (G) Fluorescence-labeled Dextran injected into the heart of xBic-C-MO1+_2_-injected Xenopus embryos is excreted via the pronephros. Arrow indicates the cloaca. en, endoderm; no, notochord; nt, neural tube; pn, pronephros; so, somite.
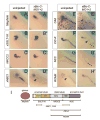
Figure 4. Expression Analysis of Pronephric Markers
(A – H’) Whole mount in situ hybridization analysis of uninjected control and xBic-C-MO1+_2_-injected embryos at stage 38. (A,A’) Nephrin; (B,B’) xSGLT-1K; (C,C’) NKCC2; (D,D’) xNBC1; (E,E’) CAII; (F,F’) ClC-K; (G,G’) NCC; (H,H’) ROMK. (I) Schematic diagram of the pronephric marker genes used. Arrows point to the late distal pronephric tubule, arrowheads indicate the pronephric duct.
Figure 5. Expression Analysis of Transcription Factors
(A - F’) Whole mount in situ hybridization analysis of uninjected control and xBic-C-MO1+_2_-injected embryos at stage 35. (A,A’) GATA-3; (B,B’) HNF1β; (C,C’) Lim-1; (D,D’) Pax-2; (E,E’) Pax-8; (F,F’) WT-1. Note that the expression of Lim-1 in the late distal tubule is lost upon microinjection of xBic-C-MO1+2 (indicated by arrowhead in C, C’). (G, H) Rescue of NBC1 expression in the late distal tubule in xBic-C-MO1+_2_-injected embryos with a single injection (2 ng) of xBic-C*, xBic-C-ΔKH, and xBic-C-ΔSAM mRNA. (G) Transverse section showing expression of NBC1 in the early proximal tubule (ept) and the late distal tubule (ldt). Note that the expression of NBC1 in the late distal tubule could only be detected on the side injected with xBic-C* mRNA. (H) Quantification of the expression of NBC1 in the late distal tubule; black - bilateral expression; white - no expression; gray - unilateral expression rescued by co-injected mRNA. Each of the rescue experiments was performed at least three independent times and the graph depicts the cumulative numbers of all experiments.
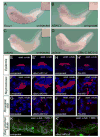
Figure 6. Bicaudal-C and Cilia Formation
(A - D) Whole mount in situ hybridization of uninjected (A - C) or xBic-C-MO1+_2_-injected Xenopus embryos (D) at stage 36 using (A) FoxJ1, (B) Axonemal Dynein Heavy Chain 9 (ADHC9) and (C, D) polaris. (E - J’) Immunocytochemistry using anti acetylated α-tubulin antibody (red) on uninjected control, xBic-C-MO1+2 and _xPol-MO_-injected embryos at stage 42. (E,E’,H,H’) Ciliated cells of the epidermis; (F,F’,I,I’) cilia of the nephrostomes; (G,G’,J,J’) individual primary cilia present on the cells of the pronephric duct. (K,K’) Immunocytochemistry using anti acetylated α-tubulin (red) and anti-NBC1 (green) antibodies on uninjected control and xBic-C-MO1+_2_-injected embryos at stage 42. Images were obtained by confocal microscopy; nuclei were counterstained with DAPI (blue).
Similar articles
- Patterning the embryonic kidney: BMP signaling mediates the differentiation of the pronephric tubules and duct in Xenopus laevis.
Bracken CM, Mizeracka K, McLaughlin KA. Bracken CM, et al. Dev Dyn. 2008 Jan;237(1):132-44. doi: 10.1002/dvdy.21387. Dev Dyn. 2008. PMID: 18069689 - Chordin affects pronephros development in Xenopus embryos by anteriorizing presomitic mesoderm.
Mitchell T, Jones EA, Weeks DL, Sheets MD. Mitchell T, et al. Dev Dyn. 2007 Jan;236(1):251-61. doi: 10.1002/dvdy.21014. Dev Dyn. 2007. PMID: 17106888 Free PMC article. - The RNA-binding protein bicaudal C regulates polycystin 2 in the kidney by antagonizing miR-17 activity.
Tran U, Zakin L, Schweickert A, Agrawal R, Döger R, Blum M, De Robertis EM, Wessely O. Tran U, et al. Development. 2010 Apr;137(7):1107-16. doi: 10.1242/dev.046045. Development. 2010. PMID: 20215348 Free PMC article. - Towards a molecular anatomy of the Xenopus pronephric kidney.
Brändli AW. Brändli AW. Int J Dev Biol. 1999;43(5):381-95. Int J Dev Biol. 1999. PMID: 10535314 Review. - Pronephric duct extension in amphibian embryos: migration and other mechanisms.
Drawbridge J, Meighan CM, Lumpkins R, Kite ME. Drawbridge J, et al. Dev Dyn. 2003 Jan;226(1):1-11. doi: 10.1002/dvdy.10205. Dev Dyn. 2003. PMID: 12508219 Review.
Cited by
- Knockdown of bicaudal C in zebrafish (Danio rerio) causes cystic kidneys: a nonmammalian model of polycystic kidney disease.
Bouvrette DJ, Sittaramane V, Heidel JR, Chandrasekhar A, Bryda EC. Bouvrette DJ, et al. Comp Med. 2010 Apr;60(2):96-106. Comp Med. 2010. PMID: 20412683 Free PMC article. - Modeling Renal Disease "On the Fly".
Millet-Boureima C, Porras Marroquin J, Gamberi C. Millet-Boureima C, et al. Biomed Res Int. 2018 May 31;2018:5697436. doi: 10.1155/2018/5697436. eCollection 2018. Biomed Res Int. 2018. PMID: 29955604 Free PMC article. Review. - Hyperphosphorylation of polycystin-2 at a critical residue in disease reveals an essential role for polycystin-1-regulated dephosphorylation.
Streets AJ, Wessely O, Peters DJ, Ong AC. Streets AJ, et al. Hum Mol Genet. 2013 May 15;22(10):1924-39. doi: 10.1093/hmg/ddt031. Epub 2013 Feb 5. Hum Mol Genet. 2013. PMID: 23390129 Free PMC article. - Fish and frogs: models for vertebrate cilia signaling.
Wessely O, Obara T. Wessely O, et al. Front Biosci. 2008 Jan 1;13:1866-80. doi: 10.2741/2806. Front Biosci. 2008. PMID: 17981674 Free PMC article. Review. - Lymph node metastasis related gene BICC1 promotes tumor progression by promoting EMT and immune infiltration in pancreatic cancer.
Meng F, Hua S, Chen X, Meng N, Lan T. Meng F, et al. BMC Med Genomics. 2023 Oct 25;16(1):263. doi: 10.1186/s12920-023-01696-4. BMC Med Genomics. 2023. PMID: 37880742 Free PMC article.
References
- Barr MM. Caenorhabditis elegans as a model to study renal development and disease: sexy cilia. J Am Soc Nephrol. 2005;16:305–312. - PubMed
- Barr MM, DeModena J, Braun D, Nguyen CQ, Hall DH, Sternberg PW. The Caenorhabditis elegans autosomal dominant polycystic kidney disease gene homologs lov-1 and pkd-2 act in the same pathway. Curr Biol. 2001;11:1341–1346. - PubMed
- Bisgrove BW, Snarr BS, Emrazian A, Yost HJ. Polaris and Polycystin-2 in dorsal forerunner cells and Kupffer’s vesicle are required for specification of the zebrafish left-right axis. Dev Biol. 2005;287:274–288. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources