Sequential chemotactic and phagocytic activation of human polymorphonuclear neutrophils - PubMed (original) (raw)
Sequential chemotactic and phagocytic activation of human polymorphonuclear neutrophils
Jens Martin Herrmann et al. Infect Immun. 2007 Aug.
Abstract
Human polymorphonuclear neutrophils (PMN) chemotax to a foreign entity. When the chemoattractants' origins are reached, specific receptors bind to the invader's surface, initiating phagocytosis, phagosome formation, and fusion with granule membranes, generating the bactericidal oxidative burst, and releasing lytic enzymes, specific peptides, and proteins. We explored the initial signaling involved in these functions by observing naïve, unprimed PMN in suspension using fluorescent indicators of cytoplasmic signals (Delta[Ca(2+)](i) and DeltapH(i)) and of bactericidal entities (oxidative species and elastase) exposed to N-formyl-methionyl-leucyl-phenylalanine (fMLP) and/or multivalent immune complexes (IC). fMLP and IC each initiate a rapid transient rise in [Ca(2+)](i), mostly from intracellular stores, simultaneously with a drop in pH(i); these are followed by a drop in [Ca(2+)](i) and a rise in pH(i), with the latter being due to a Na(+)/H(+) antiport. The impact of a second stimulation depends on the order in which stimuli are applied, on their dose, and on their nature. Provided that [Ca(2+)](i) is restored, 10(-7) M fMLP, previously shown to elicit maximal Delta[Ca(2+)](i) but no bactericidal functions, did not prevent the cells' responses with Delta[Ca(2+)](i) to a subsequent high dose of fMLP or IC; conversely, cells first exposed to 120 mug/ml IC, previously shown to elicit maximal Delta[Ca(2+)](i) and bactericidal functions, exhibited no subsequent Delta[Ca(2+)](i) or DeltapH(i) to either stimulus. While exposure to 10(-7) M fMLP, which saturates the PMN high-affinity receptor, did not elicit bactericidal release from these naïve unprimed PMN in suspension, 10(-5) M fMLP did, presumably via the low-affinity receptor, using a different Ca(2+) source.
Figures
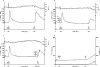
FIG. 1.
Sequential stimulation of PMN by fPR1 (high-affinity)-saturating doses (10−7 M) of fMLP 5 min apart. (a) Control; (b) 5 mM EGTA added 15 s before the first fMLP injection; (c) 5 mM EGTA added before the second fMLP injection; (d) release of elastase (shown as F460) and of oxidative products (shown as F530). Each figure is representative of six independent experiments. In this and each of the following figures, the [Ca2+]i and pHi are indicated on the ordinates; F460 is proportional to the elastase release, and F530 is proportional to the release of oxidative products.
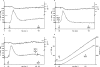
FIG. 2.
Sequential stimulation of PMN by high doses (10−5 M) of fMLP, followed 5 min later by 10−7 M fMLP. (a) Control; (b) 5 mM EGTA added 15 s before the first fMLP injection; (e) control and oxidative product release (see Materials and Methods); (f) 10−5 M fMLP followed by 120 μg/ml IC. (c and d) Reversal of the order of sequential stimulation, 10−7 M fMLP, followed by 10−5 M fMLP 5 min later. (c) Control; (d) 5 mM EGTA added 15 s before the first fMLP injection. Each figure is representative of four independent experiments.
FIG. 3.
Sequential stimulation of PMN by saturating doses (10−7 M) of fMLP followed by 120 μg/ml IC 5 min later. (a) Control; (b) 5 mM EGTA added 15 s before fMLP injection; (c) 5 mM EGTA added before IC injection; (d) release of elastase (shown as F460) and of oxidative products (shown as F530). Each figure is representative of five independent experiments.
FIG. 4.
Sequential stimulation of PMN by saturating doses (120 μg/ml) of IC followed 5 min later by 10−7 M fMLP. (a) Control; (b) 5 mM EGTA added 15 s before IC injection; (c) 5 mM EGTA added before fMLP injection; (d) release of elastase (shown as F460) and of oxidative products (shown as F530). Each figure is representative of five independent experiments.
FIG. 5.
Sequential stimulation of PMN by saturating doses (120 μg/ml) of IC 5 min apart. (a) Control, (b) 5 mM EGTA added 15 s before the first IC injection; (c) 5 mM EGTA added before the second IC injection; (d) release of elastase (shown as F460) and of oxidative products (shown as F530). In panel d, the sharp decrease upon injection is due to light scatter (see Results). Each figure is representative of six independent experiments.
Similar articles
- A cytosolic calcium transient is not necessary for degranulation or oxidative burst in immune complex-stimulated neutrophils.
Seetoo KF, Schonhorn JE, Gewirtz AT, Zhou MJ, McMenamin ME, Delva L, Simons ER. Seetoo KF, et al. J Leukoc Biol. 1997 Sep;62(3):329-40. doi: 10.1002/jlb.62.3.329. J Leukoc Biol. 1997. PMID: 9307071 - Antibodies directed to the chemotactic factor receptor detect differences between chemotactically normal and defective neutrophils from LJP patients.
DeNardin E, DeLuca C, Levine MJ, Genco RJ. DeNardin E, et al. J Periodontol. 1990 Oct;61(10):609-17. doi: 10.1902/jop.1990.61.10.609. J Periodontol. 1990. PMID: 2231227 - Tissue injury and inflammation induced by immune complexes: the critical role of the neutrophil leukocyte.
Movat HZ. Movat HZ. Exp Mol Pathol. 1979 Aug;31(1):201-10. doi: 10.1016/0014-4800(79)90021-2. Exp Mol Pathol. 1979. PMID: 222602 Review. No abstract available.
Cited by
- The neutrophil in vascular inflammation.
Phillipson M, Kubes P. Phillipson M, et al. Nat Med. 2011 Nov 7;17(11):1381-90. doi: 10.1038/nm.2514. Nat Med. 2011. PMID: 22064428 Free PMC article. Review. - Neutrophil migration under normal and sepsis conditions.
Lerman YV, Kim M. Lerman YV, et al. Cardiovasc Hematol Disord Drug Targets. 2015;15(1):19-28. doi: 10.2174/1871529x15666150108113236. Cardiovasc Hematol Disord Drug Targets. 2015. PMID: 25567338 Free PMC article. Review. - Cytokine induced expression of programmed death ligands in human neutrophils.
Bankey PE, Banerjee S, Zucchiatti A, De M, Sleem RW, Lin CF, Miller-Graziano CL, De AK. Bankey PE, et al. Immunol Lett. 2010 Apr 8;129(2):100-7. doi: 10.1016/j.imlet.2010.01.006. Epub 2010 Feb 1. Immunol Lett. 2010. PMID: 20123111 Free PMC article. - Oxidant Sensing by TRPM2 Inhibits Neutrophil Migration and Mitigates Inflammation.
Wang G, Cao L, Liu X, Sieracki NA, Di A, Wen X, Chen Y, Taylor S, Huang X, Tiruppathi C, Zhao YY, Song Y, Gao X, Jin T, Bai C, Malik AB, Xu J. Wang G, et al. Dev Cell. 2016 Sep 12;38(5):453-62. doi: 10.1016/j.devcel.2016.07.014. Epub 2016 Aug 25. Dev Cell. 2016. PMID: 27569419 Free PMC article. - Measurement of phagocytosis and of the phagosomal environment in polymorphonuclear phagocytes by flow cytometry.
Simons ER. Simons ER. Curr Protoc Cytom. 2010 Jan;Chapter 9:Unit9.31. doi: 10.1002/0471142956.cy0931s51. Curr Protoc Cytom. 2010. PMID: 20069529 Free PMC article.
References
- Bernardo, J., A. M. Billingslea, M. F. Ortiz, K. F. Seetoo, J. Macauley, and E. R. Simons. 1997. Adherence-dependent calcium signaling in monocytes: induction of a CD14-high phenotype, stimulus-responsive subpopulation. J. Immunol. Methods 209:165-175. - PubMed
- Bernardo, J., H. Hartlaub, X. Yu, H. Long, and E. R. Simons. 2002. Immune complex stimulation of human neutrophils involves a novel Ca2+ /H+ exchanger that participates in the regulation of cytoplasmic pH: flow cytometric analysis of Ca2+/pH responses by subpopulations. J. Leukoc. Biol. 72:1172-1179. - PubMed
- Brown, G. E., M. Q. Stewart, S. A. Bissonnette, A. E. Elia, E. Wilker, and M. B. Yaffe. 2004. Distinct ligand-dependent roles for p38 MAPK in priming and activation of the neutrophil NADPH oxidase. J. Biol. Chem. 279:27059-27068. - PubMed
- Brunkhorst, B. A., G. Strohmeier, K. Lazzari, G. Weil, D. Melnick, H. B. Fleit, and E. R. Simons. 1992. Differential roles of Fc gamma RII and Fc gamma RIII in immune complex stimulation of human neutrophils. J. Biol. Chem. 267:20659-20666. - PubMed
- Brunkhorst, B. A., K. G. Lazzari, G. Strohmeier, G. Weil, and E. R. Simons. 1991. Calcium changes in immune complex-stimulated human neutrophils. Simultaneous measurement of receptor occupancy and activation reveals full population stimulus binding but subpopulation activation. J. Biol. Chem. 266:13035-13043. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- RR 00533/RR/NCRR NIH HHS/United States
- M01 RR000533/RR/NCRR NIH HHS/United States
- HL 76463/HL/NHLBI NIH HHS/United States
- P50 DE016191/DE/NIDCR NIH HHS/United States
- DE 16191/DE/NIDCR NIH HHS/United States
- R01 HL076463/HL/NHLBI NIH HHS/United States
- DK 31056/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous