A mutation in the myostatin gene increases muscle mass and enhances racing performance in heterozygote dogs - PubMed (original) (raw)
A mutation in the myostatin gene increases muscle mass and enhances racing performance in heterozygote dogs
Dana S Mosher et al. PLoS Genet. 2007.
Abstract
Double muscling is a trait previously described in several mammalian species including cattle and sheep and is caused by mutations in the myostatin (MSTN) gene (previously referred to as GDF8). Here we describe a new mutation in MSTN found in the whippet dog breed that results in a double-muscled phenotype known as the "bully" whippet. Individuals with this phenotype carry two copies of a two-base-pair deletion in the third exon of MSTN leading to a premature stop codon at amino acid 313. Individuals carrying only one copy of the mutation are, on average, more muscular than wild-type individuals (p = 7.43 x 10(-6); Kruskal-Wallis Test) and are significantly faster than individuals carrying the wild-type genotype in competitive racing events (Kendall's nonparametric measure, tau = 0.3619; p approximately 0.00028). These results highlight the utility of performance-enhancing polymorphisms, marking the first time a mutation in MSTN has been quantitatively linked to increased athletic performance.
Conflict of interest statement
Competing interests. The authors have declared that no competing interests exist.
Figures
Figure 1. Comparison of Whippets with Each of the Three Potential Genotypes
(A) Dogs have two copies of the wild-type allele (+/+). (B) Dogs are heterozygous with one wild-type allele and one mutant cys → stop allele (mh/+). (C) Dogs are homozygous for the mutant allele with two copies of the cys → stop mutation (mh/mh). All photos represent unique individuals except for the top and middle panels in the righthand column.
Figure 2. A 2-bp Deletion at Nucleotides 939 and 940 of the Canine MSTN Coding Sequence
This deletion results in a cysteine→stop codon change at amino acid 313. The top panel shows the sequence trace of a wild-type (+/+) individual in the region of the mutation. The middle panel shows the sequence trace of a mh/+ individual with a single copy of both the wild-type and mutant alleles. The bottom panel shows the sequence trace from a homozygote “bully” dog of the mh/mh genotype. The amino acid sequences for +/+ and mh/mh individuals are located above each trace.
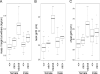
Figure 3. Variation in Musculature among Whippets of the Three Potential Genotypes
Whippets homozygous for the cys → stop mutation have a higher mass-to-height ratio (A), a larger neck girth (B), and larger chest girth (C) than wild-type or heterozygous individuals. Males and females are shown separately. +/+, wild-type individuals; mh/+, individuals heterozygous for the cys → stop mutation; and mh/mh, individuals homozygous for the cys → stop mutation. The center bar indicates the median value and the top and bottom edges of the box delimit the 75th and 25th percentiles, respectively.
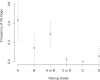
Figure 4. Frequency of Whippets Carrying the Mutation among the Dogs Sampled in Each Racing Grade
Dotted lines above and below each point represent 95% confidence intervals on the frequency (± 1.96 standard error on the mean).
Figure 5. Haplotypes Spanning the MSTN Gene Interval from 15 Dog Breeds
SNPs and indels were genotyped from sequence reads aligned by Phrap. Insertions and deletions are indicated by I and D, respectively. Haplotypes are shown on the left side with alleles colored yellow if they match the golden jackal, or blue otherwise. The golden jackal is missing data for markers at positions 3,698,667 and 3,698,824 so the most common allele was assumed to be the ancestral allele and was colored yellow. Marker positions and the MSTN gene are shown to scale along Chromosome 37 in the plot at the top left. The table to the right shows the number of chromosomes inferred to carry the haplotype in any given breed. Haplotypes were inferred by PHASE independently for each breed and the most likely haplotype pair was selected for each individual. The mh mutation (nucleotide position 3,692,430 circled in red) occurs on just one haplotype and is observed only in the whippet breed.
Similar articles
- Sprinting without myostatin: a genetic determinant of athletic prowess.
Lee SJ. Lee SJ. Trends Genet. 2007 Oct;23(10):475-7. doi: 10.1016/j.tig.2007.08.008. Epub 2007 Sep 19. Trends Genet. 2007. PMID: 17884234 - Targeted mutations in myostatin by zinc-finger nucleases result in double-muscled phenotype in Meishan pigs.
Qian L, Tang M, Yang J, Wang Q, Cai C, Jiang S, Li H, Jiang K, Gao P, Ma D, Chen Y, An X, Li K, Cui W. Qian L, et al. Sci Rep. 2015 Sep 24;5:14435. doi: 10.1038/srep14435. Sci Rep. 2015. PMID: 26400270 Free PMC article. - Myostatin and its implications on animal breeding: a review.
Bellinge RH, Liberles DA, Iaschi SP, O'brien PA, Tay GK. Bellinge RH, et al. Anim Genet. 2005 Feb;36(1):1-6. doi: 10.1111/j.1365-2052.2004.01229.x. Anim Genet. 2005. PMID: 15670124 Review. - Genetic variation in the bovine myostatin gene in UK beef cattle: allele frequencies and haplotype analysis in the South Devon.
Smith JA, Lewis AM, Wiener P, Williams JL. Smith JA, et al. Anim Genet. 2000 Oct;31(5):306-9. doi: 10.1046/j.1365-2052.2000.00521.x. Anim Genet. 2000. PMID: 11105210 - Mutations in the myostatin gene leading to hypermuscularity in mammals: indications for a similar mechanism in fish?
Stinckens A, Georges M, Buys N. Stinckens A, et al. Anim Genet. 2011 Jun;42(3):229-34. doi: 10.1111/j.1365-2052.2010.02144.x. Epub 2010 Dec 22. Anim Genet. 2011. PMID: 21175702 Review.
Cited by
- Sex-based limits to running speed in the human, horse and dog: The role of sexual dimorphisms.
Senefeld JW, Shepherd JRA, Baker SE, Joyner MJ. Senefeld JW, et al. FASEB J. 2021 May;35(5):e21562. doi: 10.1096/fj.202100161R. FASEB J. 2021. PMID: 33913189 Free PMC article. Review. - Genomics and genetics in the biology of adaptation to exercise.
Bouchard C, Rankinen T, Timmons JA. Bouchard C, et al. Compr Physiol. 2011 Jul;1(3):1603-48. doi: 10.1002/cphy.c100059. Compr Physiol. 2011. PMID: 23733655 Free PMC article. Review. - Muscle-specific transgenic expression of porcine myostatin propeptide enhances muscle growth in mice.
Wang K, Li Z, Li Y, Zeng J, He C, Yang J, Liu D, Wu Z. Wang K, et al. Transgenic Res. 2013 Oct;22(5):1011-9. doi: 10.1007/s11248-013-9709-4. Epub 2013 Mar 30. Transgenic Res. 2013. PMID: 23543410 - Similar sequences but dissimilar biological functions of GDF11 and myostatin.
Suh J, Lee YS. Suh J, et al. Exp Mol Med. 2020 Oct;52(10):1673-1693. doi: 10.1038/s12276-020-00516-4. Epub 2020 Oct 19. Exp Mol Med. 2020. PMID: 33077875 Free PMC article. Review. - Role of Skeletal Muscle in Insulin Resistance and Glucose Uptake.
Merz KE, Thurmond DC. Merz KE, et al. Compr Physiol. 2020 Jul 8;10(3):785-809. doi: 10.1002/cphy.c190029. Compr Physiol. 2020. PMID: 32940941 Free PMC article. Review.
References
- Ostrander EA, Comstock KE. The domestic dog genome. Curr Biol. 2004;14:R98–R99. - PubMed
- Sutter NB, Ostrander EA. Dog star rising: The canine genetic system. Nat Rev Genet. 2004;5:900–910. - PubMed
- American Kennel Club. The Complete Dog Book. 19th Edition. New York: Howell Book House; 1998. 790
- Szabo G, Dallmann G, Muller G, Patthy L, Soller M, et al. A deletion in the myostatin gene causes the compact (Cmpt) hypermuscular mutation in mice. Mamm Genome. 1998;9:671–672. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous