Mecp2 deficiency leads to delayed maturation and altered gene expression in hippocampal neurons - PubMed (original) (raw)
Mecp2 deficiency leads to delayed maturation and altered gene expression in hippocampal neurons
Richard D Smrt et al. Neurobiol Dis. 2007 Jul.
Abstract
It is well known that Rett Syndrome, a severe postnatal childhood neurological disorder, is mostly caused by mutations in the MECP2 gene. However, how deficiencies in MeCP2 contribute to the neurological dysfunction of Rett Syndrome is not clear. We aimed to resolve the role of MeCP2 epigenetic regulation in postnatal brain development in an Mecp2-deficient mouse model. We found that, while Mecp2 was not critical for the production of immature neurons in the dentate gyrus (DG) of the hippocampus, the newly generated neurons exhibited pronounced deficits in neuronal maturation, including delayed transition into a more mature stage, altered expression of presynaptic proteins and reduced dendritic spine density. Furthermore, analysis of gene expression profiles of isolated DG granule neurons revealed abnormal expression levels of a number of genes previously shown to be important for synaptogenesis. Our studies suggest that MeCP2 plays a central role in neuronal maturation, which might be mediated through epigenetic control of expression pathways that are instrumental in both dendritic development and synaptogenesis.
Figures
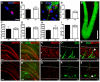
Figure 1. Mecp2 KO mice exhibit a normal early stage of postnatal neurogenesis even though immature neurons have an abnormal morphology
(A) For cell proliferation analyses, NSCs isolated from 6-week-old KO and WT brains were cultured in the presence of BrdU to label dividing cells. BrdU-labeled cells were detected by immunocytochemistry (red, BrdU; blue: DAPI nuclear staining; scale bar=10 μm). (B) Quantitative analyses of BrdU-labeled cells indicated no significant difference in cell proliferation between KO and WT NSCs in vitro (p=0.41, n=3, t-test). (C) Mecp2 KO NSCs can differentiate into neurons (TuJI+, red) and astrocytes (S100b+, green) (blue: DAPI nuclear staining); scale bar=10 μm. (D) There was no significant difference in neuronal differentiation between KO and WT NSCs in vitro (p=0.43, n=3 t-test). (E) Example of a brain section stained with antibodies to NeuN (green) and BrdU (red) for in vivo neurogenesis analyses. Neither 4-week-old (F) nor 8-week-old (G) KO mice exhibited significant deficits in the number of BrdU+ cells at either 1 day post-BrdU injection (F and G) or 4 weeks post-BrdU injection (H). At 4 weeks post-labeling, BrdU+ KO cells differentiated into similar numbers of new neurons (I) compared to WT mice. Low magnification (J-M, scale bar=100 μm) and high magnification (N-S, scale bar=10 μm) images of DG stained with antibodies against Mecp2 (red nuclear staining) and DCX (green). Note that Mecp2 staining is absent in KO brains (L, M, Q-S). (N-S) DCX+ immature neurons in KO brains have disorganized morphologies compared to those in WT brains (arrowhead in P), with abnormal orientation of the processes of many DCX+ neurons (arrowhead in S). The dotted lines in N-S indicate the boundary of the granule cell layer. m, molecular layer, g, granule cell layer, and h, hilar region.
Figure 2. Immature neurons in the DG of Mecp2 KO mice have delayed transitioning to mature stage
(A-D) Confocal images showing DG of the hippocampus labeled with antibodies against NeuN (A, green, mature neurons), DCX (B, red, immature neurons), and DAPI (C, blue, nuclear dye). Scale bars=100 μm. (D) Merged image of A-C. (E-G) Higher magnification images of granule neurons and examples of neurons that are NeuN+DCX− (asterisk) and NeuN+DCX+ (arrowhead). Scale bars=10 μm. (H) Quantitative analyses indicate the percentage of mature neurons (NeuN+DCX−, white bar), immature neurons (DCX+NeuN−, light gray) and “transitioning neurons” (NeuN+DCX+, dark gray) in 4-week-old and 8-week-old mice. (I) Age-dependent changes in the proportion of “transitioning neurons” over total neurons are significantly different between KO and WT mice (p<0.0001), (J) The percentage of “transitioning neurons” among total DCX+ neurons are also significantly different between WT and KO mice at both 4 and 8-weeks-old. While WT mice exhibited an age-dependent decrease in the proportion of “transitioning neurons” over total DCX+ neurons, KO mice displayed an increase (p<0.0001), suggesting more neurons are stalled at the transitioning stage in the KO brains.
Figure 3. Mecp2 KO mice have altered presynaptic protein expression pattern
(A-C) Digitized bright-field micrographs show synaptophysin immunoreactivity in the adult and young mice. Scale bar=100 μm. (A) The box indicates the region that is enlarged in C and D. (B) Sections were incubated with normal rabbit IgG, instead of synaptophysin antibody, as a negative control. (C) Example of a brain section with small synaptophysin positive spots (arrowheads) and two large clusters (arrows). scale bar=10 μm. (D) The output of particle analysis of (C) produced by Image-J showing the two large clusters (arrows). (E) Number of large clusters in 4- and 8-week-old WT mice (**P < 0.01) compared to 4- and 8-week-old KO mice. Note that WT animals showed a clear age-dependent reduction in the density of large synapse clusters, whereas KO failed to show this developmental change.
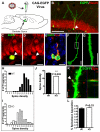
Figure 4. Newly matured neurons in Mecp2 KO hippocampus have reduced dendritic spine density and abnormal distribution
(A) Schematic diagram demonstrating stereotaxic grafting of CAG-eGFP retrovirus into the DG of 4-week-old KO and WT mice to label dividing neuroprogenitors in the germinal zone of the DG. At 4 weeks post grafting, many eGFP+ cells had differentiated into NeuN+ (B, C, D, arrowheads) and/or DCX+ (E) new neurons. (F, G) High resolution image of dendrites of eGFP+ neurons were used to quantify the density of dendritic spines (number of spines/10 μm dendrites) to generate the data in (H-J). (G) High magnification view of the box in F. Scale bars in F and G=10 μm. (J) New neurons in KO brains had reduced dendritic spine density (P<0.005, n=3 t-test). (H, I) Frequency distribution data indicate higher variation in spine density in KO mice (I) than in WT mice (H), indicating an uneven distribution of spine density. (K) Z-stack confocal image showing apposition of presynaptic terminal marker synaptophysin (red, arrow) with eGFP+ spines of new neurons. (L) Quantitative analyses indicating that similar percentages of eGFP+ spines were apposed to presynaptic terminals in both WT and Mecp2 KO mice (p=0.33, n=3, t-test)
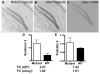
Figure 5. Gene expression analyses of LCM-isolated DG neurons indicate altered expression of genes related to synaptogenesis
(A-C) Bright field images demonstrate the process of isolating DG granule neurons from cresyl violet-stained brain sections using LCM. (C) Isolated neurons (dark) were melted into the cap during the LCM procedure and used for RNA isolation. (D-E) Real time PCR analyses confirmed the differential expression of two of the candidate genes, Syndecan 2 and Prefoldin 5. The fold changes determined by real time PCR are consistent with those determined by microarray analyses (p<0.05; n=3 experiments with 4 mice/genotype).
Similar articles
- Dendritic spine pathologies in hippocampal pyramidal neurons from Rett syndrome brain and after expression of Rett-associated MECP2 mutations.
Chapleau CA, Calfa GD, Lane MC, Albertson AJ, Larimore JL, Kudo S, Armstrong DL, Percy AK, Pozzo-Miller L. Chapleau CA, et al. Neurobiol Dis. 2009 Aug;35(2):219-33. doi: 10.1016/j.nbd.2009.05.001. Epub 2009 May 12. Neurobiol Dis. 2009. PMID: 19442733 Free PMC article. - Neural development of methyl-CpG-binding protein 2 null embryonic stem cells: a system for studying Rett syndrome.
Okabe Y, Kusaga A, Takahashi T, Mitsumasu C, Murai Y, Tanaka E, Higashi H, Matsuishi T, Kosai K. Okabe Y, et al. Brain Res. 2010 Nov 11;1360:17-27. doi: 10.1016/j.brainres.2010.08.090. Epub 2010 Sep 25. Brain Res. 2010. PMID: 20816763 - Partial rescue of MeCP2 deficiency by postnatal activation of MeCP2.
Giacometti E, Luikenhuis S, Beard C, Jaenisch R. Giacometti E, et al. Proc Natl Acad Sci U S A. 2007 Feb 6;104(6):1931-6. doi: 10.1073/pnas.0610593104. Epub 2007 Jan 31. Proc Natl Acad Sci U S A. 2007. PMID: 17267601 Free PMC article. - MeCP2 expression and function during brain development: implications for Rett syndrome's pathogenesis and clinical evolution.
Kaufmann WE, Johnston MV, Blue ME. Kaufmann WE, et al. Brain Dev. 2005 Nov;27 Suppl 1:S77-S87. doi: 10.1016/j.braindev.2004.10.008. Epub 2005 Sep 22. Brain Dev. 2005. PMID: 16182491 Review. - Rett syndrome: insights into genetic, molecular and circuit mechanisms.
Ip JPK, Mellios N, Sur M. Ip JPK, et al. Nat Rev Neurosci. 2018 Jun;19(6):368-382. doi: 10.1038/s41583-018-0006-3. Nat Rev Neurosci. 2018. PMID: 29740174 Free PMC article. Review.
Cited by
- Cell cloning-based transcriptome analysis in Rett patients: relevance to the pathogenesis of Rett syndrome of new human MeCP2 target genes.
Nectoux J, Fichou Y, Rosas-Vargas H, Cagnard N, Bahi-Buisson N, Nusbaum P, Letourneur F, Chelly J, Bienvenu T. Nectoux J, et al. J Cell Mol Med. 2010 Jul;14(7):1962-74. doi: 10.1111/j.1582-4934.2010.01107.x. Epub 2010 Jun 21. J Cell Mol Med. 2010. PMID: 20569274 Free PMC article. - Epigenetic choreographers of neurogenesis in the adult mammalian brain.
Ma DK, Marchetto MC, Guo JU, Ming GL, Gage FH, Song H. Ma DK, et al. Nat Neurosci. 2010 Nov;13(11):1338-44. doi: 10.1038/nn.2672. Nat Neurosci. 2010. PMID: 20975758 Free PMC article. Review. - Stem cell-derived neurons from autistic individuals with SHANK3 mutation show morphogenetic abnormalities during early development.
Kathuria A, Nowosiad P, Jagasia R, Aigner S, Taylor RD, Andreae LC, Gatford NJF, Lucchesi W, Srivastava DP, Price J. Kathuria A, et al. Mol Psychiatry. 2018 Mar;23(3):735-746. doi: 10.1038/mp.2017.185. Epub 2017 Sep 26. Mol Psychiatry. 2018. PMID: 28948968 Free PMC article. - Cross talk between microRNA and epigenetic regulation in adult neurogenesis.
Szulwach KE, Li X, Smrt RD, Li Y, Luo Y, Lin L, Santistevan NJ, Li W, Zhao X, Jin P. Szulwach KE, et al. J Cell Biol. 2010 Apr 5;189(1):127-41. doi: 10.1083/jcb.200908151. J Cell Biol. 2010. PMID: 20368621 Free PMC article. - Reduced synaptic activity in neuronal networks derived from embryonic stem cells of murine Rett syndrome model.
Barth L, Sütterlin R, Nenniger M, Vogt KE. Barth L, et al. Front Cell Neurosci. 2014 Mar 26;8:79. doi: 10.3389/fncel.2014.00079. eCollection 2014. Front Cell Neurosci. 2014. PMID: 24723848 Free PMC article.
References
- Aimone JB, Gage FH. Unbiased characterization of high-density oligonucleotide microarrays using probe-level statistics. J Neurosci Methods. 2004;135:27–33. - PubMed
- Aimone JB, Leasure JL, Perreau VM, Thallmair M. Spatial and temporal gene expression profiling of the contused rat spinal cord. Exp Neurol. 2004;189:204–221. - PubMed
- Akbarian S, Chen RZ, Gribnau J, Rasmussen TP, Fong H, Jaenisch R, Jones EG. Expression pattern of the Rett syndrome gene MeCP2 in primate prefrontal cortex. Neurobiol Dis. 2001;8:784–791. - PubMed
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl- CpG-binding protein 2. Nat Genet. 1999;23:185–188. - PubMed
- Armstrong DD. Neuropathology of Rett syndrome. Ment Retard Dev Disabil Res Rev. 2002;8:72–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P20 RR015636-086248/RR/NCRR NIH HHS/United States
- T34 GM008751/GM/NIGMS NIH HHS/United States
- R01 MH080434/MH/NIMH NIH HHS/United States
- P20 RR015636-076866/RR/NCRR NIH HHS/United States
- P20 RR015636/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases