Restoration of p53 expression in human cancer cell lines upregulates the expression of Notch1: implications for cancer cell fate determination after genotoxic stress - PubMed (original) (raw)
Restoration of p53 expression in human cancer cell lines upregulates the expression of Notch1: implications for cancer cell fate determination after genotoxic stress
Fatouma Alimirah et al. Neoplasia. 2007 May.
Abstract
Following genotoxic stress, transcriptional activation of target genes by p53 tumor suppressor is critical in cell fate determination. Here we report that the restoration of p53 function in human cancer cell lines that are deficient in p53 function upregulated the expression of Notch1. Interestingly, the expression of wild-type p53 in human prostate and breast cancer cell lines correlated well with increased expression of Notch1. Furthermore, knockdown of p53 expression in cancer cells that express wild-type p53 resulted in reduced expression of Notch1. Importantly, genotoxic stress to cancer cells that resulted in activation of p53 also upregulated the expression of Notch1. Moreover, p53-mediated induction of Notch1 expression was associated with stimulation of the activity of Notch-responsive reporters. Notably, p53 differentially regulated the expression of Notch family members: expression of Notch2 and Notch4 was not induced by p53. Significantly, treatment of cells with gamma secretase inhibitor, an inhibitor of Notch signaling, increased susceptibility to apoptosis in response to genotoxic stress. Together, our observations suggest that p53-mediated upregulation of Notch1 expression in human cancer cell lines contributes to cell fate determination after genotoxic stress.
Figures
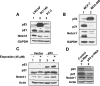
Figure 1
Restoration of p53 expression in the human Saos-2 osteosarcoma cell line upregulates the expression of Notch1. (A) Subconfluent cultures of SaosArg72 cells were either incubated at 39°C (lane 1), incubated at 32°C for 16 hours (lane 2), or incubated at 32°C for 24 hours (lane 3). After incubation, total cell lysates were analyzed by immunoblotting using antibodies specific to the indicated proteins. (B) Saos-2 cells were nucleofected with either an empty vector pCMV (lane 1) or the plasmid pCMV-p53 encoding wild-type p53 (lane 2). Sixty hours after the nucleofection of cells, cells were lysed, and cell lysates containing an equal amount of proteins were processed for immunoblotting using antibodies specific to the indicated proteins. (C) Subconfluent cultures of SaosArg72 cells were incubated either at 39°C (lane 1) or at 32°C (lane 2). Twenty-four hours after incubation, total RNA was isolated, and steady-state levels of Notch1, p21, and actin mRNA were analyzed by semiquantitative reverse transcription-polymerase chain reaction. (D and E) Two sets of SaosArg72 cell cultures (in 60-mm plates) were transfected with p21-luc, CBF1-luc, or Hes1-luc reporter plasmid (1.8 µg) along with pRL-TK plasmid (0.2 ag; plasmids in a 9:1 ratio) using FuGene6 transfection reagent. One set of plates for each reporter was incubated at 39°C, and the other sets of plates were incubated at 32°C. Forty-four hours after incubation, cells were processed for dual luciferase reporter activity assays, as described in Materials and Methods. Firefly luciferase reporter activity was normalized to Renilla luciferase activity to control for variations in nucleofection efficiencies. Luciferase activity for p21-luc (C) and for CBF1-luc and Hes1-luc (D) in control cells is shown.
Figure 2
The functional status of p53 and its expression levels in human cancer cell lines correlate with expression levels of Notch1 protein. (A) Total cell lysates from human prostate cancer cell lines LNCaP (lane 1), DU-145 (lane 2), or PC-3 (lane 3) were analyzed by immunoblotting using antibodies specific to the indicated proteins. (B) Total cell lysates from the human breast cancer cell line MCF-7 (lane 1) or MDA-468 (lane 2) were analyzed by immunoblotting using antibodies specific to the indicated proteins. (C) PC-3 cells were nucleofected with either pCMV vector (2 µg; lanes 1 and 2) or pCMV-p53 (wild-type) plasmid (2 µg; lanes 3 and 4), as described in Materials and Methods. Twenty-four hours after nucleofection, cells were either left untreated (lanes 1 and 3) or treated with etoposide (45 M) for 24 hours. Forty-eight hours after nucleofection, total cell extracts were analyzed by immunoblotting using antibodies specific to the indicated proteins. (D) LNCaP cells were transfected with either control siRNA (lane 1) or a pool of p53 siRNA (lane 2), as described in Materials and Methods, using Lipofectamine transfection reagent. Sixty hours after the transfection of cells, total cell lysates were analyzed by immunoblotting using antibodies specific to the indicated proteins.
Figure 3
Etoposide treatment-induced genotoxic stress in p53-positive human cancer cell lines upregulates Notch1 expression. (A) Total cell lysates from the human prostate cancer cell line LNCaP, treated with either DMSO alone (lane 1), 45 µM etoposide (final concentration; lane 2), or 90 µM etoposide (final concentration; lane 3) for 15 hours, were analyzed by immunoblotting using antibodies specific to the indicated proteins. (B) Total cell lysates from the human breast cancer cell line MCF-7, either treated with DMSO alone (lane 1) or treated with 45 µM etoposide (final concentration) for the indicated time (hours), were analyzed by immunoblotting using antibodies specific to the indicated proteins. (C and D) Total cell lysates from the human prostate cancer cell line PC-3 (C) or DU-145 (D), either treated with DMSO alone (lane 1) or treated with indicated concentrations of etoposide for 24 hours, were analyzed by immunoblotting using antibodies specific to the indicated proteins.
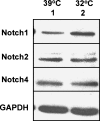
Figure 4
p53 differentially regulates the expression of Notch receptor family members. Subconfluent cultures of SaosArg72 cells were incubated at either 39°C (lane 1) or 32°C (lane 2) for 24 hours. After incubation, total cell lysates were analyzed by immunoblotting using antibodies specific to the indicated proteins.
Figure 5
Inhibition of Notch1 activity and genotoxic stress increase susceptibility to apoptosis. (A) Subconfluent cultures of MCF-7 cell line were either left untreated (lane 1) or treated with GSI (lane 2; 25 µM), etoposide (lane 3; 45 µM), or both GSI and etoposide (lane 4). Cells were incubated for 20 hours, and total cell lysates were analyzed by immunoblotting using antibodies specific to the indicated proteins. Two arrows indicate two forms of PARP protein: the precursor form (113 kDa) and the cleaved form (85 kDa). (B) Subconfluent cultures of the MCF-7 cell line were either left untreated (control; top left panel) or treated with GSI (25 µM; top right panel), etoposide (45 µM; bottom left panel), or both GSI and etoposide (bottom right panel). After incubation of cells for 20 hours, floating and attached cells were collected and processed for propidium iodide staining followed by flow cytometry. The percentage of cells in sub-G0 phase is indicated in each panel.
Similar articles
- Regulation of Notch1 gene expression by p53 in epithelial cells.
Yugawa T, Handa K, Narisawa-Saito M, Ohno S, Fujita M, Kiyono T. Yugawa T, et al. Mol Cell Biol. 2007 May;27(10):3732-42. doi: 10.1128/MCB.02119-06. Epub 2007 Mar 12. Mol Cell Biol. 2007. PMID: 17353266 Free PMC article. - DeltaNp63alpha repression of the Notch1 gene supports the proliferative capacity of normal human keratinocytes and cervical cancer cells.
Yugawa T, Narisawa-Saito M, Yoshimatsu Y, Haga K, Ohno S, Egawa N, Fujita M, Kiyono T. Yugawa T, et al. Cancer Res. 2010 May 15;70(10):4034-44. doi: 10.1158/0008-5472.CAN-09-4063. Epub 2010 May 4. Cancer Res. 2010. PMID: 20442293 - p53-dependent and -independent regulation of the death receptor KILLER/DR5 gene expression in response to genotoxic stress and tumor necrosis factor alpha.
Sheikh MS, Burns TF, Huang Y, Wu GS, Amundson S, Brooks KS, Fornace AJ Jr, el-Deiry WS. Sheikh MS, et al. Cancer Res. 1998 Apr 15;58(8):1593-8. Cancer Res. 1998. PMID: 9563466 - Notch tumor suppressor function.
Dotto GP. Dotto GP. Oncogene. 2008 Sep 1;27(38):5115-23. doi: 10.1038/onc.2008.225. Oncogene. 2008. PMID: 18758480 Free PMC article. Review. - Activation of the Notch1 Stem Cell Signaling Pathway during Routine Cell Line Subculture.
Liu W, Morgan KM, Pine SR. Liu W, et al. Front Oncol. 2014 Aug 6;4:211. doi: 10.3389/fonc.2014.00211. eCollection 2014. Front Oncol. 2014. PMID: 25147757 Free PMC article. Review. No abstract available.
Cited by
- Differentiation of the ductal epithelium and smooth muscle in the prostate gland are regulated by the Notch/PTEN-dependent mechanism.
Wu X, Xu K, Zhang L, Deng Y, Lee P, Shapiro E, Monaco M, Makarenkova HP, Li J, Lepor H, Grishina I. Wu X, et al. Dev Biol. 2011 Aug 15;356(2):337-49. doi: 10.1016/j.ydbio.2011.05.659. Epub 2011 May 20. Dev Biol. 2011. PMID: 21624358 Free PMC article. - Notch activation by phenethyl isothiocyanate attenuates its inhibitory effect on prostate cancer cell migration.
Kim SH, Sehrawat A, Sakao K, Hahm ER, Singh SV. Kim SH, et al. PLoS One. 2011;6(10):e26615. doi: 10.1371/journal.pone.0026615. Epub 2011 Oct 24. PLoS One. 2011. PMID: 22039516 Free PMC article. - The War on Cancer rages on.
Rehemtulla A. Rehemtulla A. Neoplasia. 2009 Dec;11(12):1252-63. doi: 10.1593/neo.91866. Neoplasia. 2009. PMID: 20019833 Free PMC article. - Notch signaling proteins: legitimate targets for cancer therapy.
Wang Z, Li Y, Sarkar FH. Wang Z, et al. Curr Protein Pept Sci. 2010 Sep;11(6):398-408. doi: 10.2174/138920310791824039. Curr Protein Pept Sci. 2010. PMID: 20491628 Free PMC article. Review. - The sphingosine kinase inhibitor 2-(p-hyroxyanilino)-4-(p-chlorophenyl)thiazole reduces androgen receptor expression via an oxidative stress-dependent mechanism.
Tonelli F, Alossaimi M, Williamson L, Tate RJ, Watson DG, Chan E, Bittman R, Pyne NJ, Pyne S. Tonelli F, et al. Br J Pharmacol. 2013 Mar;168(6):1497-505. doi: 10.1111/bph.12035. Br J Pharmacol. 2013. PMID: 23113536 Free PMC article.
References
- Ko J, Prives C. p53: puzzle and paradigm. Genes Dev. 1996;10:1054–1072. - PubMed
- Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. - PubMed
- Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. - PubMed
- Vousden KH, Lu X. Live or let die: the cell's response to p53. Nat Rev Cancer. 2002;2:594–604. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous