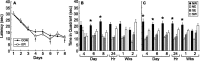Plant-derived flavanol (-)epicatechin enhances angiogenesis and retention of spatial memory in mice - PubMed (original) (raw)
Comparative Study
Plant-derived flavanol (-)epicatechin enhances angiogenesis and retention of spatial memory in mice
Henriette van Praag et al. J Neurosci. 2007.
Erratum in
- J Neurosci. 2007 Aug 1;27(31):ii
Abstract
Diet and exercise have a profound impact on brain function. In particular, natural nutrients found in plants may influence neuronal survival and plasticity. Here, we tested whether consumption of a plant-derived flavanol, (-)epicatechin, enhances cognition in sedentary or wheel-running female C57BL/6 mice. Retention of spatial memory in the water maze was enhanced by ingestion of (-)epicatechin, especially in combination with exercise. Improved spatial memory was associated with increased angiogenesis and neuronal spine density, but not newborn cell survival, in the dentate gyrus of the hippocampus. Moreover, microarray analysis showed upregulation of genes associated with learning and downregulation of markers of neurodegeneration in the hippocampus. Together, our data show that ingestion of a single flavanol improves spatial memory retention in adult mammals.
Figures
Figure 1.
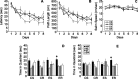
Water maze learning in mice fed control or (−)epicatechin food pellets and housed with or without access to a running wheel (2 h per day) for 42 d. Mice were trained for 8 d with two trials per day between days 31 and 38 to find the hidden platform in the Morris water maze. A–C, Latency (A), path length (B), and swim speed (C) did not differ between the groups. D, E, The probe tests (40 s) at 4 h (D) and 24 h (E) after the last trial on day 8 showed that ER mice spent significantly more time in the target quadrant (NW) than any of the other quadrants (*p < 0.05). Error bars indicate SEM. NW, Northwest; NE, northeast; SE, southeast; SW, southwest.
Figure 2.
Spatial learning in mice that received control (CON) or (−)epicatechin (EPI) food pellets for 14 d. Thereafter, mice were fed control diet for the duration of study. Animals were trained in the water maze with four trials per day over 8 d between days 21 and 28. A, Acquisition of the task did not differ between the groups. B, C, Retention of the task was assessed using probe trials on days 4, 6, and 8 of training, as well as 24 h, 1 week, and 2 weeks after the last training session in mice fed CON (B) or EPI (C) diet. On day 4 of training, only the EPI group (C) showed a significant preference for the platform quadrant (NW; *p < 0.05). On days 6 and 8, the control (B) and (−)epicatechin (C) groups searched the target zone (NW) more than other quadrants (*p < 0.05). At 24 h and 1 week after the last training session, only the EPI mice (C) still preferred the platform quadrant (*p < 0.05). Error bars indicate SEM. NW, Northwest; NE, northeast; SE, southeast; SW, southwest.
Figure 3.
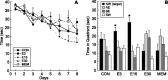
Dose–response of (−)epicatechin and comparison with memantine: effects on water maze learning. Mice were given (−)epicatechin (0, 3, 15, or 30 mg/d) or memantine (0.6 mg/d) in drinking water for 3 weeks. A, Mice were trained in the Morris water maze with four trials per day for 8 d between days 23 and 30. Acquisition of the task did not differ between control (CON), 3 mg (E3), 15 mg (E15), and 30 mg (E30) of (−)epicatechin or memantine (MEM). B, Retention of the task was significantly improved in the E3 and E15 mice. In the 60 s probe trial on day 8, the E3 and E15 groups spent more time in the target area (NW) than in the other quadrants (p < 0.03), whereas CON, E30, and MEM mice did not show a significant preference for the NW quadrant. Error bars indicate SEM. NW, Northwest; NE, northeast; SE, southeast; SW, southwest.
Figure 4.
Vasculature in the DG of mice that consumed control or (−)epicatechin diet. Lectin-stained vessels (red) were quantified using imaging software to assign a pseudocolor mask (yellow) to highlight the threshold for brightness and luma. There was a significant increase in vasculature in the DG of flavanol-treated mice (B, D) compared with controls (A, C). The outline of the analyzed area is marked in all panels and includes the DG and molecular layer.
Figure 5.
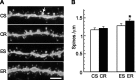
DiI labeling of DG granule cells in mice that received control or (−)epicatechin diet under sedentary or running conditions for 6 weeks. A, Representative images of DiI-labeled dendritic fragments in the outer molecular layer of the DG in CS, CR, ES, and ER mice. The arrow indicates a mushroom spine of which the estimated surface area is no less than 0.4 μm2. Scale bar, 5 μm. B, Spine-density quantification showed that neurons from ER mice had more spines than cells from CS (*p < 0.014) or CR (*p < 0.03) animals. Error bars indicate SEM.
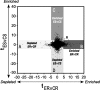
Figure 6.
Two-dimensional representation of gene expression changes resulting from 6 weeks of daily (−)epicatechin consumption with or without running. t statistics and cutoffs (p < 0.005) for ER versus CR (ERvCR; _x_-axis) and ES versus CS (ESvCS; _y_-axis) mice were computed as described in Materials and Methods. Each (x, y) point in the graph represents the t statistic (_t_ER,CR, _t_ES,CS) for a probe set. A–D, Specifically, genes that are depleted (A) or enriched (B) in ER are shown in the horizontal axis; enriched (C) or depleted (D) gene expression with (−)epicatechin alone (ES) is represented in the vertical axis. e, Four genes that are uniquely enriched in both sedentary and running flavanol-treated mice.
Similar articles
- Endurance factors improve hippocampal neurogenesis and spatial memory in mice.
Kobilo T, Yuan C, van Praag H. Kobilo T, et al. Learn Mem. 2011 Jan 18;18(2):103-7. doi: 10.1101/lm.2001611. Print 2011 Feb. Learn Mem. 2011. PMID: 21245211 Free PMC article. - Functional analysis of neurovascular adaptations to exercise in the dentate gyrus of young adult mice associated with cognitive gain.
Clark PJ, Brzezinska WJ, Puchalski EK, Krone DA, Rhodes JS. Clark PJ, et al. Hippocampus. 2009 Oct;19(10):937-50. doi: 10.1002/hipo.20543. Hippocampus. 2009. PMID: 19132736 Free PMC article. - Angiogenesis but not neurogenesis is critical for normal learning and memory acquisition.
Kerr AL, Steuer EL, Pochtarev V, Swain RA. Kerr AL, et al. Neuroscience. 2010 Nov 24;171(1):214-26. doi: 10.1016/j.neuroscience.2010.08.008. Epub 2010 Sep 8. Neuroscience. 2010. PMID: 20804819 - Exercise prevents high-fat diet-induced impairment of flexible memory expression in the water maze and modulates adult hippocampal neurogenesis in mice.
Klein C, Jonas W, Iggena D, Empl L, Rivalan M, Wiedmer P, Spranger J, Hellweg R, Winter Y, Steiner B. Klein C, et al. Neurobiol Learn Mem. 2016 May;131:26-35. doi: 10.1016/j.nlm.2016.03.002. Epub 2016 Mar 8. Neurobiol Learn Mem. 2016. PMID: 26968656 - Spatial navigation: implications for animal models, drug development and human studies.
Stuchlik A, Kubik S, Vlcek K, Vales K. Stuchlik A, et al. Physiol Res. 2014;63(Suppl 1):S237-49. doi: 10.33549/physiolres.932660. Physiol Res. 2014. PMID: 24564663 Review.
Cited by
- Chocolate, Air Pollution and Children's Neuroprotection: What Cognition Tools should be at Hand to Evaluate Interventions?
Calderón-Garcidueñas L, San Juan Chávez V, Vacaseydel-Aceves NB, Calderón-Sánchez R, Macías-Escobedo E, Frías C, Giacometto M, Velasquez L, Félix-Villarreal R, Martin JD, Draheim C, Engle RW. Calderón-Garcidueñas L, et al. Front Pharmacol. 2016 Aug 11;7:232. doi: 10.3389/fphar.2016.00232. eCollection 2016. Front Pharmacol. 2016. PMID: 27563291 Free PMC article. Review. - Neuroprotective Effects of Açaí (Euterpe oleracea Mart.) against Rotenone In Vitro Exposure.
Machado AK, Andreazza AC, da Silva TM, Boligon AA, do Nascimento V, Scola G, Duong A, Cadoná FC, Ribeiro EE, da Cruz IB. Machado AK, et al. Oxid Med Cell Longev. 2016;2016:8940850. doi: 10.1155/2016/8940850. Epub 2016 Oct 3. Oxid Med Cell Longev. 2016. PMID: 27781077 Free PMC article. - Cognitive Function and Consumption of Fruit and Vegetable Polyphenols in a Young Population: Is There a Relationship?
Carrillo JÁ, Zafrilla MP, Marhuenda J. Carrillo JÁ, et al. Foods. 2019 Oct 17;8(10):507. doi: 10.3390/foods8100507. Foods. 2019. PMID: 31627296 Free PMC article. Review. - Pharmacological Manipulation of Trk, p75NTR, and NGF Balance Restores Memory Deficit in Global Ischemia/Reperfusion Model in Rats.
Choucry AM, Al-Shorbagy MY, Attia AS, El-Abhar HS. Choucry AM, et al. J Mol Neurosci. 2019 May;68(1):78-90. doi: 10.1007/s12031-019-01284-1. Epub 2019 Mar 12. J Mol Neurosci. 2019. PMID: 30863991 - Recent Advances in Microbiota-Associated Metabolites in Heart Failure.
Masenga SK, Povia JP, Lwiindi PC, Kirabo A. Masenga SK, et al. Biomedicines. 2023 Aug 21;11(8):2313. doi: 10.3390/biomedicines11082313. Biomedicines. 2023. PMID: 37626809 Free PMC article. Review.
References
- Abd El Mohsen MM, Kuhnle G, Rechner AR, Schroeter H, Rose S, Jenner P, Rice-Evans CA. Uptake and metabolism of epicatechin and its access to the brain after oral ingestion. Free Radical Biol Med. 2002;33:1693–1702. - PubMed
- Adayev T, Chen-Hwang MC, Murakami N, Wegiel J, Hwang YW. Kinetic properties of a MNB/DYRK1A mutant suitable for the elucidation of biochemical pathways. Biochemistry. 2006;45:12011–12019. - PubMed
- Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9:723–727. - PubMed
- Barnes CA, Danysz W, Parsons CG. Effects of the uncompetitive NMDA receptor antagonist memantine on hippocampal long-term potentiation, short-term exploratory modulation and spatial memory in awake, freely moving rats. Eur J Neurosci. 1996;8:565–571. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical