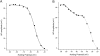Inactivation properties of sodium channel Nav1.8 maintain action potential amplitude in small DRG neurons in the context of depolarization - PubMed (original) (raw)
Inactivation properties of sodium channel Nav1.8 maintain action potential amplitude in small DRG neurons in the context of depolarization
T Patrick Harty et al. Mol Pain. 2007.
Abstract
Background: Small neurons of the dorsal root ganglion (DRG) express five of the nine known voltage-gated sodium channels. Each channel has unique biophysical characteristics which determine how it contributes to the generation of action potentials (AP). To better understand how AP amplitude is maintained in nociceptive DRG neurons and their centrally projecting axons, which are subjected to depolarization within the dorsal horn, we investigated the dependence of AP amplitude on membrane potential, and how that dependence is altered by the presence or absence of sodium channel Nav1.8.
Results: In small neurons cultured from wild type (WT) adult mouse DRG, AP amplitude decreases as the membrane potential is depolarized from -90 mV to -30 mV. The decrease in amplitude is best fit by two Boltzmann equations, having V1/2 values of -73 and -37 mV. These values are similar to the V1/2 values for steady-state fast inactivation of tetrodotoxin-sensitive (TTX-s) sodium channels, and the tetrodotoxin-resistant (TTX-r) Nav1.8 sodium channel, respectively. Addition of TTX eliminates the more hyperpolarized V1/2 component and leads to increasing AP amplitude for holding potentials of -90 to -60 mV. This increase is substantially reduced by the addition of potassium channel blockers. In neurons from Nav1.8(-/-) mice, the voltage-dependent decrease in AP amplitude is characterized by a single Boltzmann equation with a V1/2 value of -55 mV, suggesting a shift in the steady-state fast inactivation properties of TTX-s sodium channels. Transfection of Nav1.8(-/-) DRG neurons with DNA encoding Nav1.8 results in a membrane potential-dependent decrease in AP amplitude that recapitulates WT properties.
Conclusion: We conclude that the presence of Nav1.8 allows AP amplitude to be maintained in DRG neurons and their centrally projecting axons even when depolarized within the dorsal horn.
Figures
Figure 1
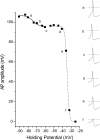
The voltage dependent decrease in AP amplitude recorded from a small diameter, WT DRG neuron is best fit by 2 Boltzmann equations. APs were elicited by threshold stimulation while holding the cell membrane potential between -90 and -25 mV. Inset waveforms on the right represent responses elicited at the membrane holding potential indicated by the small letters (a-f). The dotted lines in the insets indicate 0 mV. The solid and dashed lines in the main figure represent Boltzmann equation fits with V1/2 values of -74.3 mV and -34.7 mV, respectively. For this cell, the contribution of the more hyperpolarized component accounted for 10% of the decrease in AP amplitude.
Figure 2
Block of TTX-s sodium channels alters the voltage dependent decrease in AP amplitude. Responses from a single small DRG neuron in the presence of 300 nM TTX (inset waveforms a-f on the right) demonstrate that AP amplitude increases with depolarization of membrane holding potential from -90 to -60 mV (inset waveforms a-c). From holding potentials of -60 to -25, AP amplitude decreases with depolarization (inset waveforms c-f), and this decrease can be fit with a single Boltzmann equation (dashed line in the main figure, V1/2 = -36.8 mV). The dotted lines in inset waveforms indicate 0 mV.
Figure 3
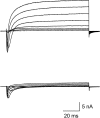
Potassium channel blockers reduce outward currents. Voltage clamped currents in response to depolarizing voltage steps from a holding potential of -100 mV are shown for two separate small DRG neurons. Cells were recorded in the absence (top) and presence (bottom) of the potassium channel blockers TEA and 4-AP.
Figure 4
Potassium channel blockers reduce the increase in AP amplitude observed in the presence of TTX for hyperpolarized holding potentials. Responses from a single small DRG neuron (inset waveforms a-f on the right) demonstrate that the increase in AP amplitude observed in the presence of TTX with depolarization of membrane holding potential from -90 to -60 mV (inset waveforms a-c) is much smaller in the presence of TEA and 4-AP. In contrast, the decrease in AP amplitude with depolarization from holding potentials of -60 to -25 mV (inset waveforms c-f) is unchanged in the presence of TEA and 4-AP. The decrease can be fit with a single Boltzmann equation (dashed line in main figure, V1/2 = -38.8 mV). The dotted lines in inset waveforms indicate 0 mV.
Figure 5
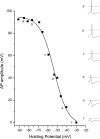
The absence of Nav1.8 sodium channels alters the voltage dependent decrease in AP amplitude. Responses from a small DRG neuron cultured from a Nav1.8(-/-) mouse are shown as inset waveforms on the right (a-f). In these neurons, the decrease in AP amplitude as membrane holding potential is depolarized from -90 to -30 mV is best fit by a single Boltzmann equation (solid line in main figure). The V1/2 (-53.4 mV for this cell) is about 20 mV more depolarized than the TTX-s component in small DRG neurons from WT mice. The dotted lines in inset waveforms indicate 0 mV.
Figure 6
Transfection with Nav1.8 produces two populations of cells with respect to voltage-dependence of AP amplitude. Data are from two small DRG neurons from Nav1.8(-/-) mice transfected with Nav1.8. The cell in (A) is representative of a population of cells (N = 7) for which the decrease in AP amplitude was best fit with single Boltzmann equation having a V1/2 of -53.9 mV (solid line). The cell in (B) is representative of a population of cells (N = 8) for which the decrease in AP amplitude was best fit by two Boltzmann equations, with V1/2 values of -72.6 mV (solid line) and -33.3 mV (dashed line).
Similar articles
- Distinct repriming and closed-state inactivation kinetics of Nav1.6 and Nav1.7 sodium channels in mouse spinal sensory neurons.
Herzog RI, Cummins TR, Ghassemi F, Dib-Hajj SD, Waxman SG. Herzog RI, et al. J Physiol. 2003 Sep 15;551(Pt 3):741-50. doi: 10.1113/jphysiol.2003.047357. Epub 2003 Jul 3. J Physiol. 2003. PMID: 12843211 Free PMC article. - Contactin regulates the current density and axonal expression of tetrodotoxin-resistant but not tetrodotoxin-sensitive sodium channels in DRG neurons.
Rush AM, Craner MJ, Kageyama T, Dib-Hajj SD, Waxman SG, Ranscht B. Rush AM, et al. Eur J Neurosci. 2005 Jul;22(1):39-49. doi: 10.1111/j.1460-9568.2005.04186.x. Eur J Neurosci. 2005. PMID: 16029194 - Differential effect of D623N variant and wild-type Na(v)1.7 sodium channels on resting potential and interspike membrane potential of dorsal root ganglion neurons.
Ahn HS, Vasylyev DV, Estacion M, Macala LJ, Shah P, Faber CG, Merkies IS, Dib-Hajj SD, Waxman SG. Ahn HS, et al. Brain Res. 2013 Sep 5;1529:165-77. doi: 10.1016/j.brainres.2013.07.005. Epub 2013 Jul 11. Brain Res. 2013. PMID: 23850641 - Roles of Voltage-Gated Tetrodotoxin-Sensitive Sodium Channels NaV1.3 and NaV1.7 in Diabetes and Painful Diabetic Neuropathy.
Yang L, Li Q, Liu X, Liu S. Yang L, et al. Int J Mol Sci. 2016 Sep 5;17(9):1479. doi: 10.3390/ijms17091479. Int J Mol Sci. 2016. PMID: 27608006 Free PMC article. Review. - Molecular diversity of structure and function of the voltage-gated Na+ channels.
Ogata N, Ohishi Y. Ogata N, et al. Jpn J Pharmacol. 2002 Apr;88(4):365-77. doi: 10.1254/jjp.88.365. Jpn J Pharmacol. 2002. PMID: 12046980 Review.
Cited by
- The functional consequences of sodium channel NaV 1.8 in human left ventricular hypertrophy.
Ahmad S, Tirilomis P, Pabel S, Dybkova N, Hartmann N, Molina CE, Tirilomis T, Kutschka I, Frey N, Maier LS, Hasenfuss G, Streckfuss-Bömeke K, Sossalla S. Ahmad S, et al. ESC Heart Fail. 2019 Feb;6(1):154-163. doi: 10.1002/ehf2.12378. Epub 2018 Oct 30. ESC Heart Fail. 2019. PMID: 30378291 Free PMC article. - Sodium channel NaV1.9 mutations associated with insensitivity to pain dampen neuronal excitability.
Huang J, Vanoye CG, Cutts A, Goldberg YP, Dib-Hajj SD, Cohen CJ, Waxman SG, George AL Jr. Huang J, et al. J Clin Invest. 2017 Jun 30;127(7):2805-2814. doi: 10.1172/JCI92373. Epub 2017 May 22. J Clin Invest. 2017. PMID: 28530638 Free PMC article. - Atypical changes in DRG neuron excitability and complex pain phenotype associated with a Nav1.7 mutation that massively hyperpolarizes activation.
Huang J, Mis MA, Tanaka B, Adi T, Estacion M, Liu S, Walker S, Dib-Hajj SD, Waxman SG. Huang J, et al. Sci Rep. 2018 Jan 29;8(1):1811. doi: 10.1038/s41598-018-20221-7. Sci Rep. 2018. PMID: 29379075 Free PMC article. - TRPA1 contributes to specific mechanically activated currents and sensory neuron mechanical hypersensitivity.
Brierley SM, Castro J, Harrington AM, Hughes PA, Page AJ, Rychkov GY, Blackshaw LA. Brierley SM, et al. J Physiol. 2011 Jul 15;589(Pt 14):3575-93. doi: 10.1113/jphysiol.2011.206789. Epub 2011 May 9. J Physiol. 2011. PMID: 21558163 Free PMC article. - Genetic variation in SCN10A influences cardiac conduction.
Chambers JC, Zhao J, Terracciano CM, Bezzina CR, Zhang W, Kaba R, Navaratnarajah M, Lotlikar A, Sehmi JS, Kooner MK, Deng G, Siedlecka U, Parasramka S, El-Hamamsy I, Wass MN, Dekker LR, de Jong JS, Sternberg MJ, McKenna W, Severs NJ, de Silva R, Wilde AA, Anand P, Yacoub M, Scott J, Elliott P, Wood JN, Kooner JS. Chambers JC, et al. Nat Genet. 2010 Feb;42(2):149-52. doi: 10.1038/ng.516. Epub 2010 Jan 10. Nat Genet. 2010. PMID: 20062061
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources