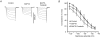Ca2+-stimulated adenylyl cyclase isoform AC1 is preferentially expressed in guinea-pig sino-atrial node cells and modulates the I(f) pacemaker current - PubMed (original) (raw)
Ca2+-stimulated adenylyl cyclase isoform AC1 is preferentially expressed in guinea-pig sino-atrial node cells and modulates the I(f) pacemaker current
Paul Mattick et al. J Physiol. 2007.
Abstract
Ca(2+)-stimulated adenylyl cyclases (AC) are known to play important roles in neurons but have not previously been reported in the heart. Here we present the first evidence for selective expression of Ca(2+)-stimulated AC in the sino-atrial node (SAN) but not in ventricular muscle of the guinea-pig heart. The AC1 isoform of Ca(2+)-stimulated AC was shown to be present in SAN, both as mRNA using RT-PCR and as protein using immuno-blotting with a specific antibody. Confocal immuno-fluorescence studies detected membrane localization of AC1 in SAN cells, but no AC1 in ventricular muscle. Ca(2+)-stimulated AC8 may also be present in SAN. The functional importance of AC activity was investigated by monitoring activation of I(f) (gated by hyperpolarization and regulated by cAMP, which shifts activation to more depolarized voltages). Basal activity of AC in isolated SAN myocytes was demonstrated by the observations that an inhibitor of AC activity (MDL 12330A, 10 microm) shifted activation in the hyperpolarizing direction, while inhibition of phosphodiesterases (IBMX, 100 microm) shifted I(f) activation in the depolarizing direction. Buffering cytosolic Ca(2+) with the Ca(2+) chelator BAPTA (by exposure to BAPTA-AM) shifted activation of I(f) in the hyperpolarizing direction, and under these conditions the AC inhibitor MDL had little or no further effect. The actions of BAPTA were overcome by exposure to forskolin (10 microm), a direct stimulator of all AC isoforms, to restore cAMP levels. These effects are consistent with the functional importance of Ca(2+)-stimulated AC, which is expected to be fundamental to initiation and regulation of the heartbeat.
Figures
Figure 1. RT-PCR and immuno-blot identify Ca2+ sensitive ACs in SAN cells
A, RT-PCR of cardiac tissue: SAN (S), atrium (A) and ventricle (V), negative control (NC) and positive control (PC) with consensus primers designed to recognize AC1, AC8, AC5 and β-actin in other mammalian species. A range of different AC8 primers (1–4) were tested in the SAN without effect, despite PC finding the experiment to be effective. B, immuno-blot of cardiac tissue using polyclonal antibodies raised against peptide sequences in AC1, AC5/6 and AC8 common to other mammalian species. Brain extracts, cerebellum (C) and hippocampus (H), were also used to test for AC1 and AC8. Molecular mass markers are shown at the left. The asterisks draw attention to specific DNA or protein bands.
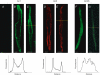
Figure 2. Ca2+ sensitive AC1 and AC8 are present on SAN plasma membranes
Localization of AC1, AC8 and AC5/6 in a single plane (_z_-axis resolution of 800 nm) of SAN cells using immuno-fluorescence and confocal microscopy. Ventricular, atrial and SAN cells were identified according to cellular morphology and size. Fluorescent labelling in ventricular (A), atrial (B, D and F) and SAN cardiac myocytes (C, E and G) and fluorescence intensity profile from SAN cells (at dashed yellow line, see inset below). Secondary antibody for AC1 and AC5/6 was AlexaFluor 488 while Rhodamine Red-X was used for AC8. Note the peripheral fluorescence in AC1 and AC8 in both atrial and SAN cells, presumably associated with the plasma membrane; however, no such staining was found in either cell type with AC5/6 or in ventricular cells with AC1. Scale bar is 5 μm.
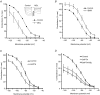
Figure 3. Effects of MDL and IBMX on basal AC activity: BAPTA and MDL inhibition of _I_f are not additive
Example _I_f currents are shown inset. MDL (10 μ
m
, ^) shifted activation (_V_1/2) in the hyperpolarizing direction (A); IBMX (100 μ
m
, ^) shifted activation in the depolarizing direction (B); BAPTA (5 μ
m
BAPTA-AM for 5 min, ^) shifted activation in the hyperpolarizing direction (C) and MDL (▵) had little or no further effect after BAPTA (D).
Figure 4. Forskolin reverses BAPTA inhibition of _I_f
After superfusion of BAPTA-AM (5 μ
m
for 5 min, ^), forskolin (10 μ
m
) increased _I_f (A, example records) and shifted activation in the depolarizing direction (▵), overcoming the effects of BAPTA (B).
Similar articles
- IP3-mediated Ca2+ release regulates atrial Ca2+ transients and pacemaker function by stimulation of adenylyl cyclases.
Capel RA, Bose SJ, Collins TP, Rajasundaram S, Ayagama T, Zaccolo M, Burton RB, Terrar DA. Capel RA, et al. Am J Physiol Heart Circ Physiol. 2021 Jan 1;320(1):H95-H107. doi: 10.1152/ajpheart.00380.2020. Epub 2020 Oct 16. Am J Physiol Heart Circ Physiol. 2021. PMID: 33064562 Free PMC article. - Autonomic modulation of sinoatrial node: Role of pacemaker current and calcium sensitive adenylyl cyclase isoforms.
Robinson RB, Dun W, Boyden PA. Robinson RB, et al. Prog Biophys Mol Biol. 2021 Nov;166:22-28. doi: 10.1016/j.pbiomolbio.2020.08.004. Epub 2020 Aug 24. Prog Biophys Mol Biol. 2021. PMID: 32853595 Free PMC article. Review. - Ca(2+)-stimulated adenylyl cyclases regulate the L-type Ca(2+) current in guinea-pig atrial myocytes.
Collins TP, Terrar DA. Collins TP, et al. J Physiol. 2012 Apr 15;590(8):1881-93. doi: 10.1113/jphysiol.2011.227066. Epub 2012 Feb 20. J Physiol. 2012. PMID: 22351635 Free PMC article. - Ca2+-activated adenylyl cyclase 1 introduces Ca2+-dependence to beta-adrenergic stimulation of HCN2 current.
Kryukova YN, Protas L, Robinson RB. Kryukova YN, et al. J Mol Cell Cardiol. 2012 Jun;52(6):1233-9. doi: 10.1016/j.yjmcc.2012.03.010. Epub 2012 Mar 29. J Mol Cell Cardiol. 2012. PMID: 22484253 Free PMC article. - Tissue specificity and physiological relevance of various isoforms of adenylyl cyclase.
Defer N, Best-Belpomme M, Hanoune J. Defer N, et al. Am J Physiol Renal Physiol. 2000 Sep;279(3):F400-16. doi: 10.1152/ajprenal.2000.279.3.F400. Am J Physiol Renal Physiol. 2000. PMID: 10966920 Review.
Cited by
- IP3-mediated Ca2+ release regulates atrial Ca2+ transients and pacemaker function by stimulation of adenylyl cyclases.
Capel RA, Bose SJ, Collins TP, Rajasundaram S, Ayagama T, Zaccolo M, Burton RB, Terrar DA. Capel RA, et al. Am J Physiol Heart Circ Physiol. 2021 Jan 1;320(1):H95-H107. doi: 10.1152/ajpheart.00380.2020. Epub 2020 Oct 16. Am J Physiol Heart Circ Physiol. 2021. PMID: 33064562 Free PMC article. - The importance of Ca(2+)-dependent mechanisms for the initiation of the heartbeat.
Capel RA, Terrar DA. Capel RA, et al. Front Physiol. 2015 Mar 25;6:80. doi: 10.3389/fphys.2015.00080. eCollection 2015. Front Physiol. 2015. PMID: 25859219 Free PMC article. Review. - The A-kinase anchoring protein Yotiao facilitates complex formation between adenylyl cyclase type 9 and the IKs potassium channel in heart.
Li Y, Chen L, Kass RS, Dessauer CW. Li Y, et al. J Biol Chem. 2012 Aug 24;287(35):29815-24. doi: 10.1074/jbc.M112.380568. Epub 2012 Jul 9. J Biol Chem. 2012. PMID: 22778270 Free PMC article. - POPDC1 scaffolds a complex of adenylyl cyclase 9 and the potassium channel TREK-1 in heart.
Baldwin TA, Li Y, Marsden AN, Rinné S, Garza-Carbajal A, Schindler RFR, Zhang M, Garcia MA, Venna VR, Decher N, Brand T, Dessauer CW. Baldwin TA, et al. EMBO Rep. 2022 Dec 6;23(12):e55208. doi: 10.15252/embr.202255208. Epub 2022 Oct 18. EMBO Rep. 2022. PMID: 36254885 Free PMC article. - Autonomic modulation of sinoatrial node: Role of pacemaker current and calcium sensitive adenylyl cyclase isoforms.
Robinson RB, Dun W, Boyden PA. Robinson RB, et al. Prog Biophys Mol Biol. 2021 Nov;166:22-28. doi: 10.1016/j.pbiomolbio.2020.08.004. Epub 2020 Aug 24. Prog Biophys Mol Biol. 2021. PMID: 32853595 Free PMC article. Review.
References
- Beaumont V, Zucker RS. Enhancement of synaptic transmission by cyclic AMP modulation of presynaptic Ih channels. Nat Neurosci. 2000;3:133–141. - PubMed
- Chang F, Gao J, Tromba C, Cohen I, DiFrancesco D. Acetylcholine reverses effects of β-agonists on pacemaker current in canine cardiac Purkinje fibers but has no direct action. A difference between primary and secondary pacemakers. Circ Res. 1990;66:633–636. - PubMed
- DiFrancesco D, Tortora P. Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature. 1991;351:145–147. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous