LIGHT, a member of the TNF superfamily, activates Stat3 mediated by NIK pathway - PubMed (original) (raw)
LIGHT, a member of the TNF superfamily, activates Stat3 mediated by NIK pathway
Nagalakshmi Nadiminty et al. Biochem Biophys Res Commun. 2007.
Abstract
Stat3, a member of the signal transducers and activators of transcription (STAT) family, is a key signal transduction protein activated by numerous cytokines, growth factors, and oncoproteins that controls cell proliferation, differentiation, development, survival, and inflammation. Constitutive activation of Stat3 has been found frequently in a wide variety of human tumors and induces cellular transformation and tumor formation. In this study, we demonstrated that LIGHT, a member of tumor necrosis factor superfamily, activates Stat3 in cancer cells. LIGHT induces dose-dependent activation of Stat3 by phosphorylation at both the tyrosine 705 and serine 727 residues. The activation of Stat3 by LIGHT appears to be mediated by NIK phosphorylation. Expression of a kinase-inactive NIK mutant abolished LIGHT induced Stat3 activation. Overexpression of an active NIK induces Stat3 activation by phosphorylation at the both tyrosine 705 and serine 727 residues. Activation of Stat3 by NIK requires NIK kinase activity as showed by kinase assays. In addition, LIGHT increases the expression of Stat3 target genes including cyclin D1, survivin, and Bcl-xL, and stimulates human LNCaP prostate cancer cell growth in vitro which can be blocked by expression of a dominant-negative Stat3 mutant. Taken together, these results indicate that in addition to activating NF-kappaB/p52, LIGHT also activates Stat3. Activation of Stat3 together with activating non-canonical NF-kappaB/p52 signaling by LIGHT may maximize its effects on cellular proliferation, survival, and inflammation.
Figures
Figure 1
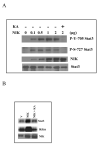
LIGHT induces Stat3 activation. A. LNCaP cells were treated with increasing doses of LIGHT as indicated for 8 h and the whole cell lysates were isolated. Twenty micrograms of protein were subjected to Western blot analysis. LIGHT increases both tyrosine and serine phosphorylation of Stat3. B. LNCaP cells were treated with 50 ng/ml of LIGHT for different time as indicated, whole cell lysates were isolated and 20 μg of protein were subjected to Western blot analysis. The phosphorylation of Stat3 at both tyrosine 705 and serine 727 by LIGHT occurs within 15 min. C. LNCaP cells were treated with increasing doses of LIGHT as indicated for 8 h and nuclear protein were isolated. Ten micrograms of the protein were subjected to EMSA. Stat3 activity was analyzed using radiolabeled probe containing consensus Stat3 DNA binding sequence as described in Materials and Methods. Oct-1 DNA binding activity was used as a control. D. LIGHT induces Stat3 phosphorylation in HEK293 cells. HEK293 cells were treated with increasing doses of LIGHT as indicated for 8 h and the whole cell lysates were isolated. Twenty micrograms of protein were subjected to Western blot analysis.
Figure 2
Blocking NIK kinase activity abolished LIHGT induced Stat3 phosphorylation. LNCaP cells were transfected with either a kinase-inactive mutant of NIK (KA) or vector control and treated with 50 ng/ml of LIGHT for 8 h. Whole cell lysates were isolated and subjected to Western blot analysis using antibodies against either tyrosine 705 Stat3 or serine 727 Stat3, or total Stat3 as a control.
Figure 3
NIK induces Stat3 phosphorylation. A. LNCaP cells were cotransfected with increasing amounts of NIK expression plasmid and the kinase inactive mutant of NIK expression plasmid (KA) as indicated. The whole cell lysates were isolated and subjected to Western blot analysis using antibodies against either tyrosine 705 Stat3 or serine 727 Stat3, or total Stat3 as a control. NIK levels show that the transfection of NIK plasmids express NIK protein in cells. B. Kinase assay to show the phosphorylation of Stat3 and IKKα by NIK. Lysates from vector, NIK, and NIK plus KA transfected LNCaP cells were immunoprecipitated with anti-NIK antibody, and the immunoprecipitated enzyme was used to phosphorylate Stat3 and IKKα in vitro. Reactions were stopped with SDS loading buffer after 30 min, loaded on SDS/PAGE, and transferred to a nitrocellulose membrane. The phosphorylated Stat3 and IKKα were visualized by autoradiography. The membrane was reprobed with anti-NIK antibody to normalize for equal amounts of kinase in each reaction.
Figure 4
LIGHT increases LNCaP cell growth in vitro. A. LNCaP cells were transfected with either the dominant-negative mutant Stat3 (S3F) or vector (EV) and treated with 50 ng/ml of LIGHT for a period of 72 h. The cells were counted at different time points as indicated. Each point is represented as the means ± SEM of four independent experiments. * indicates significantly different (p< 0.05) from the control. B. LNCaP cells were transfected with either the dominant-negative mutant Stat3 (Stat3F) or vector (EV) and treated with 50 ng/ml of LIGHT for 72 h. The whole cell lysates were isolated and subjected to Western blot analysis. LIGHT increases the expression of cyclin D1, survivin, Bcl-xL, and phosphorylated Stat3, which was abolished by expression of the dominant-negative Stat3 mutant (Stat3F). β-actin was used as a loading control.
Similar articles
- Lipoxin A4 inhibits TNF-alpha-induced production of interleukins and proliferation of rat mesangial cells.
Wu SH, Lu C, Dong L, Zhou GP, He ZG, Chen ZQ. Wu SH, et al. Kidney Int. 2005 Jul;68(1):35-46. doi: 10.1111/j.1523-1755.2005.00379.x. Kidney Int. 2005. PMID: 15954894 - Respiratory syncytial virus influences NF-kappaB-dependent gene expression through a novel pathway involving MAP3K14/NIK expression and nuclear complex formation with NF-kappaB2.
Choudhary S, Boldogh S, Garofalo R, Jamaluddin M, Brasier AR. Choudhary S, et al. J Virol. 2005 Jul;79(14):8948-59. doi: 10.1128/JVI.79.14.8948-8959.2005. J Virol. 2005. PMID: 15994789 Free PMC article. - TNF-alpha/IL-1/NF-kappaB transduction pathway in human cancer prostate.
Royuela M, Rodríguez-Berriguete G, Fraile B, Paniagua R. Royuela M, et al. Histol Histopathol. 2008 Oct;23(10):1279-90. doi: 10.14670/HH-23.1279. Histol Histopathol. 2008. PMID: 18712680 Review. - Zinc-finger protein 91 plays a key role in LIGHT-induced activation of non-canonical NF-κB pathway.
Jin HR, Jin X, Lee JJ. Jin HR, et al. Biochem Biophys Res Commun. 2010 Oct 1;400(4):581-6. doi: 10.1016/j.bbrc.2010.08.107. Epub 2010 Sep 6. Biochem Biophys Res Commun. 2010. PMID: 20804734 - Non-canonical NF-κB signaling activation and regulation: principles and perspectives.
Razani B, Reichardt AD, Cheng G. Razani B, et al. Immunol Rev. 2011 Nov;244(1):44-54. doi: 10.1111/j.1600-065X.2011.01059.x. Immunol Rev. 2011. PMID: 22017430 Review.
Cited by
- NIK stabilization in osteoclasts results in osteoporosis and enhanced inflammatory osteolysis.
Yang C, McCoy K, Davis JL, Schmidt-Supprian M, Sasaki Y, Faccio R, Novack DV. Yang C, et al. PLoS One. 2010 Nov 8;5(11):e15383. doi: 10.1371/journal.pone.0015383. PLoS One. 2010. PMID: 21151480 Free PMC article. - Construction of NF-κB-targeting RNAi adenovirus vector and the effect of NF-κB pathway on proliferation and apoptosis of vascular endothelial cells.
Chen G, Qiao Y, Yao J, Jiang Q, Lin X, Chen F, Lin F, Lin M, Lin L, Zhu P. Chen G, et al. Mol Biol Rep. 2011 Jun;38(5):3089-94. doi: 10.1007/s11033-010-9977-5. Epub 2010 Feb 3. Mol Biol Rep. 2011. PMID: 20127516 - Functional interplay between NF-κB-inducing kinase and c-Abl kinases limits response to Aurora inhibitors in multiple myeloma.
Mazzera L, Abeltino M, Lombardi G, Cantoni AM, Ria R, Ricca M, Saltarella I, Naponelli V, Rizzi FMA, Perris R, Corradi A, Vacca A, Bonati A, Lunghi P. Mazzera L, et al. Haematologica. 2019 Dec;104(12):2465-2481. doi: 10.3324/haematol.2018.208280. Epub 2019 Apr 4. Haematologica. 2019. PMID: 30948493 Free PMC article. - Regulation of Th17 cell differentiation and EAE induction by MAP3K NIK.
Jin W, Zhou XF, Yu J, Cheng X, Sun SC. Jin W, et al. Blood. 2009 Jun 25;113(26):6603-10. doi: 10.1182/blood-2008-12-192914. Epub 2009 May 1. Blood. 2009. PMID: 19411637 Free PMC article. - IκB kinases modulate the activity of the androgen receptor in prostate carcinoma cell lines.
Jain G, Voogdt C, Tobias A, Spindler KD, Möller P, Cronauer MV, Marienfeld RB. Jain G, et al. Neoplasia. 2012 Mar;14(3):178-89. doi: 10.1593/neo.111444. Neoplasia. 2012. PMID: 22496618 Free PMC article.
References
- Mauri DN, Ebner R, Montgomery RI, Kochel KD, Cheung TC, Yu GL, Ruben S, Murphy M, Eisenberg RJ, Cohen GH, Spear PG, Ware CF. LIGHT, a new member of the TNF superfamily, and lymphotoxin alpha are ligands for herpesvirus entry mediator. Immunity. 1998;8:21–30. - PubMed
- Smith CA, Farrah T, Goodwin RG. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994;76:959–62. - PubMed
- Zhai Y, Guo R, Hsu TL, Yu GL, Ni J, Kwon BS, Jiang GW, Lu J, Tan J, Ugustus M, Carter K, Rojas L, Zhu F, Lincoln C, Endress G, Xing L, Wang S, Oh KO, Gentz R, Ruben S, Lippman ME, Hsieh SL, Yang D. LIGHT, a novel ligand for lymphotoxin beta receptor and TR2/HVEM induces apoptosis and suppresses in vivo tumor formation via gene transfer. J Clin Invest. 1998;102:1142–51. - PMC - PubMed
- Chang YH, Hsieh SL, Chen MC, Lin WW. Lymphotoxin beta receptor induces interleukin 8 gene expression via NF-kappaB and AP-1 activation. Exp Cell Res. 2002;278:166–74. - PubMed
- Mackay F, Majeau GR, Hochman PS, Browning JL. Lymphotoxin beta receptor triggering induces activation of the nuclear factor kappaB transcription factor in some cell types. J Biol Chem. 1996;271:24934–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA090271-05/CA/NCI NIH HHS/United States
- R01 CA109441/CA/NCI NIH HHS/United States
- CA109441/CA/NCI NIH HHS/United States
- CA90271/CA/NCI NIH HHS/United States
- R01 CA109441-02/CA/NCI NIH HHS/United States
- R01 CA090271/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous