Spleen tyrosine kinase Syk is necessary for E-selectin-induced alpha(L)beta(2) integrin-mediated rolling on intercellular adhesion molecule-1 - PubMed (original) (raw)
Spleen tyrosine kinase Syk is necessary for E-selectin-induced alpha(L)beta(2) integrin-mediated rolling on intercellular adhesion molecule-1
Alexander Zarbock et al. Immunity. 2007 Jun.
Abstract
Engagement of neutrophils by E-selectin results in integrin activation. Here, we investigated primary mouse neutrophils in whole blood by using intravital microscopy and autoperfused flow chambers. Slow rolling on E-selectin coimmobilized with intercellular adhesion molecule-1 (ICAM-1) required P-selectin glycoprotein ligand (PSGL)-1, was dependent on alpha(L)beta(2) integrin (LFA-1), and required continuous E-selectin engagement. Slow rolling was abolished by pharmacological blockade of spleen tyrosine kinase (Syk) and was absent in Syk(-/-) bone-marrow chimeric mice. Treatment with tumor necrosis factor-alpha lowered rolling velocity further and induced CXC chemokine ligand-1 (CXCL1) and CXC chemokine receptor-2 (CXCR2)-dependent leukocyte arrest on E-selectin and ICAM-1. Arrest but not rolling was blocked by an allosteric inhibitor of LFA-1 activation. Neutrophil recruitment in a thioglycollate-induced peritonitis model was almost completely inhibited in Selplg(-/-) mice or Syk(-/-) bone-marrow chimeras treated with pertussis toxin. This identifies a second neutrophil-activation pathway that is as important as activation through G protein-coupled receptors (GPCRs).
Figures
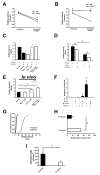
Figure 1. PSGL-1, but not CD44, is required for neutrophil slow rolling on ICAM-1 upon E-selectin engagement
Carotid cannulas were placed in untreated WT and CD44-/- mice and connected to E-selectin and E-selectin+ICAM-1-coated autoperfused flow chambers. Rolling velocity of neutrophils presented as mean ± SEM (A). Rolling velocity of neutrophils from untreated WT and PSGL-1 deficient mice on E-selectin and E-selectin+ICAM presented as mean ± SEM (B). Rolling velocity of untreated neutrophils on E-selectin+ICAM-1 before and after blocking LFA-1 or Mac-1 or both (C). (D) Rolling velocity of neutrophils from untreated mice on P-selectin and P-selectin+ICAM-1. (E) Analysis of the rolling velocity of neutrophils in inflamed cremaster venules of P-selectin-/- mice 2h after TNF-α injection. (F) Neutrophil adhesion in the flow chamber after co-immobilization of CXCL1 (10 µg/ml) with P-selectin+ICAM-1 or E-selectin+ICAM-1. The wall shear stress in all flow chamber experiments was 5.94 dynes/cm2. At least three mice and four flow chambers per group. (G-I) Intravital microscopy of the cremaster muscle from P-selectin deficient mice and E-selectin deficient mice 2h after TNF-α application. Rolling velocity (G), rolling flux fraction (H), and number of adherent cells (I).At least four mice and 20 vessels per group. * P < 0.05.
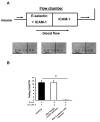
Figure 2. Continuous E-selectin engagement is required to keep LFA-1 in an activated conformation
(A) The first half of autoperfused flow chambers was coated with E-selectin+ICAM-1 and the second half with ICAM-1 alone. Cells rolled on E-selectin+ICAM-1 but detached immediately upon reaching ICAM-1 alone (representative of four independent experiments). (B) Number of rolling neutrophils on E-selectin+ICAM-1 coated chambers before and after injection of a blocking E-selectin antibody (9A9). Data presented are the mean ± SEM from at least four mice and four flow chambers (n=4). # P < 0.05.
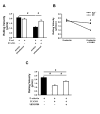
Figure 3. Syk is involved in integrin activation after E-selectin or P-selectin binding to PSGL-1
Whole blood was perfused through E-selectin and E-selectin+ICAM-1 coated autoperfused flow chambers at the indicated wall shear stress. The rolling velocity of neutrophils from mice pre-treated with piceatannol (1mg/mouse, A), a specific Syk inhibitor, and Syk-/- chimeric mice (B) were determined after 6 minutes (n=4). (C) Rolling velocity on E-selectin and E-selectin+ICAM-1 was measured after blocking of the mitogen-activated protein (MAP)-kinase p38 by the inhibitor SB203580 (100 μg/mouse i.p.) 1h prior to the experiment (n=3). # P < 0.05.
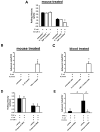
Figure 4. TNF-α, but not IL-1β, causes neutrophil arrest on E-selectin + ICAM-1
(A) Carotid cannulas of TNF-α or IL-1β pre-treated mice were connected to E-selectin and E-selectin+ICAM-1-coated autoperfused flow chambers. Rolling velocity of neutrophils was determined at a wall shear stress of 5.94 dyn/cm2 (n=4) (B, C) Number of adherent neutrophils on E-selectin+ICAM-1 of TNF-α pre-treated mice or whole blood after 6 minutes. Mice were pre-treated with TNF-α and an allosteric inhibitor (Compound 6845) of LFA-1 or vehicle and rolling velocity (D) and number of adherent neutrophils (E) were determined on E-selectin and E-selectin+ICAM-1. To ensure that only LFA-1-dependent rolling and adhesion were investigated, all mice were treated with a blocking monoclonal antibody to Mac-1 (n=4). * and # P < 0.05.
Figure 5. CXCR2 and Gαi, but not Syk, control neutrophil arrest in response to TNF-α
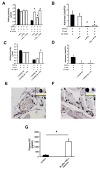
Rolling velocity of neutrophils from TNF-α pre-treated mice on E-selectin and E-selectin+ICAM-1 coated flow chambers after blocking Gαi with PTx or Syk by piceatannol alone or in combination (A). (B) Neutrophil adhesion on E-selectin+ICAM-1 coated flow chambers after blocking Gαi with PTx or Syk by piceatannol alone or in combination of TNFα pre-treated mice. Rolling velocity (C) and neutrophil adhesion (D) on E-selectin+ICAM-1 coated flow chambers after blockade of CXCR2 by antibody or of Syk by piceatannol alone or in combination following TNF-α application. Cremaster muscles of WT mice were stained for CXCL1 without (E) and with (F) TNF-α. TNF-α injection induced an increase in adherent leukocytes in the microcirculation of the cremaster muscle in WT mice compared to untreated mice. Inserts show typical neutrophils stained for CXCL1 (E, F). (G) CXCL1 concentration in plasma before and 2h after TNF-α injection (n=4). # P < 0.05.
Figure 6. Model of LFA-1 activation during neutrophil rolling
E-selectin binding to PSGL-1 leads, through Syk (arrows), to partial LFA-1 (open curved arrow) activation that supports rolling on ICAM-1 (shown as dimer). CXCR2 engagement by CXCL1 leads, through Gai, to full LFA-1 activation, resulting in arrest on ICAM-1 (filled curved arrow). Straight arrows indicate signaling pathway, but interactions may be indirect. LFA-1 conformations from Takagi et al (Takagi and Springer, 2002).
Figure 7. Inhibition of Gαi in PSGL-1-/- mice blocks neutrophil recruitment in vivo
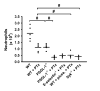
Peritoneal neutrophil influx 4 h after 1 ml injection of 4% thioglycollate into WT mice, WT mice that had received 4 μg PTx before thioglycollate injection, PSGL-1-/- mice, PSGL-1-/- mice plus PTx, E-selectin-/- mice, E-selectin-/- mice plus PTx, WT mice that had received 1mg piceatannol (picea) and 4 μg PTx, and Syk-/- chimeric mice (Syk-/-) plus 4 μg PTx (n=5 each). Data expressed as total numbers of neutrophils in the peritoneal lavage fluid counted using Kimura-stained samples, # P < 0.05.
Similar articles
- PSGL-1 engagement by E-selectin signals through Src kinase Fgr and ITAM adapters DAP12 and FcR gamma to induce slow leukocyte rolling.
Zarbock A, Abram CL, Hundt M, Altman A, Lowell CA, Ley K. Zarbock A, et al. J Exp Med. 2008 Sep 29;205(10):2339-47. doi: 10.1084/jem.20072660. Epub 2008 Sep 15. J Exp Med. 2008. PMID: 18794338 Free PMC article. - E-selectin engages PSGL-1 and CD44 through a common signaling pathway to induce integrin alphaLbeta2-mediated slow leukocyte rolling.
Yago T, Shao B, Miner JJ, Yao L, Klopocki AG, Maeda K, Coggeshall KM, McEver RP. Yago T, et al. Blood. 2010 Jul 22;116(3):485-94. doi: 10.1182/blood-2009-12-259556. Epub 2010 Mar 18. Blood. 2010. PMID: 20299514 Free PMC article. - Rolling on E- or P-selectin induces the extended but not high-affinity conformation of LFA-1 in neutrophils.
Kuwano Y, Spelten O, Zhang H, Ley K, Zarbock A. Kuwano Y, et al. Blood. 2010 Jul 29;116(4):617-24. doi: 10.1182/blood-2010-01-266122. Epub 2010 May 5. Blood. 2010. PMID: 20445017 Free PMC article. - Reprint of Neutrophil cell surface receptors and their intracellular signal transduction pathways.
Futosi K, Fodor S, Mócsai A. Futosi K, et al. Int Immunopharmacol. 2013 Dec;17(4):1185-97. doi: 10.1016/j.intimp.2013.11.010. Epub 2013 Nov 18. Int Immunopharmacol. 2013. PMID: 24263067 Review. - Neutrophil cell surface receptors and their intracellular signal transduction pathways.
Futosi K, Fodor S, Mócsai A. Futosi K, et al. Int Immunopharmacol. 2013 Nov;17(3):638-50. doi: 10.1016/j.intimp.2013.06.034. Epub 2013 Aug 30. Int Immunopharmacol. 2013. PMID: 23994464 Free PMC article. Review.
Cited by
- The extracellular cyclophilin A-integrin β2 complex as a therapeutic target of viral pneumonia.
Bai X, Yang W, Zhao Y, Cao T, Lin R, Jiao P, Li H, Li H, Min J, Jia X, Zhang H, Fan W, Jia X, Bi Y, Liu W, Sun L. Bai X, et al. Mol Ther. 2024 May 1;32(5):1510-1525. doi: 10.1016/j.ymthe.2024.03.008. Epub 2024 Mar 7. Mol Ther. 2024. PMID: 38454605 - PSGL-1, a Strategic Biomarker for Pathological Conditions in HIV Infection: A Hypothesis Review.
Zaongo SD, Chen Y. Zaongo SD, et al. Viruses. 2023 Oct 31;15(11):2197. doi: 10.3390/v15112197. Viruses. 2023. PMID: 38005875 Free PMC article. Review. - GM-CSF receptor/SYK/JNK/FOXO1/CD11c signaling promotes atherosclerosis.
Tsukui D, Kimura Y, Kono H. Tsukui D, et al. iScience. 2023 Jul 11;26(8):107293. doi: 10.1016/j.isci.2023.107293. eCollection 2023 Aug 18. iScience. 2023. PMID: 37520709 Free PMC article. - Targeting Neutrophil β2-Integrins: A Review of Relevant Resources, Tools, and Methods.
Conley HE, Sheats MK. Conley HE, et al. Biomolecules. 2023 May 26;13(6):892. doi: 10.3390/biom13060892. Biomolecules. 2023. PMID: 37371473 Free PMC article. Review. - Distinct bidirectional regulation of LFA1 and α4β7 by Rap1 and integrin adaptors in T cells under shear flow.
Kamioka Y, Ueda Y, Kondo N, Tokuhiro K, Ikeda Y, Bergmeier W, Kinashi T. Kamioka Y, et al. Cell Rep. 2023 Jun 27;42(6):112580. doi: 10.1016/j.celrep.2023.112580. Epub 2023 Jun 1. Cell Rep. 2023. PMID: 37267105 Free PMC article.
References
- Abbal C, Lambelet M, Bertaggia D, Gerbex C, Martinez M, Arcaro A, Schapira M, Spertini O. Lipid raft adhesion receptors and Syk regulate selectin-dependent rolling under flow conditions. Blood. 2006;108:3352–3359. - PubMed
- Berton G, Mocsai A, Lowell CA. Src and Syk kinases: key regulators of phagocytic cell activation. Trends Immunol. 2005;26:208–214. - PubMed
- Campbell JJ, Hedrick J, Zlotnik A, Siani MA, Thompson DA, Butcher EC. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science. 1998;279:381–384. - PubMed
- Chesnutt BC, Smith DF, Raffler NA, Smith ML, White EJ, Ley K. Induction of LFA-1-dependent neutrophil rolling on ICAM-1 by engagement of E-selectin. Microcirculation. 2006;13:99–109. - PubMed
- Crutchfield KL, Shinde Patil VR, Campbell CJ, Parkos CA, Allport JR, Goetz DJ. CD11b/CD18-coated microspheres attach to E-selectin under flow. J Leukoc Biol. 2000;67:196–205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 EB002185-06A2/EB/NIBIB NIH HHS/United States
- HL 73361/HL/NHLBI NIH HHS/United States
- R01 EB002185/EB/NIBIB NIH HHS/United States
- R01 HL054136-08/HL/NHLBI NIH HHS/United States
- P01 HL073361/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous