Agm1/Pgm3-mediated sugar nucleotide synthesis is essential for hematopoiesis and development - PubMed (original) (raw)
. 2007 Aug;27(16):5849-59.
doi: 10.1128/MCB.00802-07. Epub 2007 Jun 4.
Jennifer Antonchuk, Donald Metcalf, Phillip O Morgan, Danielle L Krebs, Jian-Guo Zhang, Douglas F Hacking, Lars Bode, Lorraine Robb, Christian Kranz, Carolyn de Graaf, Melanie Bahlo, Nicos A Nicola, Stephen L Nutt, Hudson H Freeze, Warren S Alexander, Douglas J Hilton, Benjamin T Kile
Affiliations
- PMID: 17548465
- PMCID: PMC1952135
- DOI: 10.1128/MCB.00802-07
Agm1/Pgm3-mediated sugar nucleotide synthesis is essential for hematopoiesis and development
Kylie T Greig et al. Mol Cell Biol. 2007 Aug.
Abstract
Carbohydrate modification of proteins includes N-linked and O-linked glycosylation, proteoglycan formation, glycosylphosphatidylinositol anchor synthesis, and O-GlcNAc modification. Each of these modifications requires the sugar nucleotide UDP-GlcNAc, which is produced via the hexosamine biosynthesis pathway. A key step in this pathway is the interconversion of GlcNAc-6-phosphate (GlcNAc-6-P) and GlcNAc-1-P, catalyzed by phosphoglucomutase 3 (Pgm3). In this paper, we describe two hypomorphic alleles of mouse Pgm3 and show there are specific physiological consequences of a graded reduction in Pgm3 activity and global UDP-GlcNAc levels. Whereas mice lacking Pgm3 die prior to implantation, animals with less severe reductions in enzyme activity are sterile, exhibit changes in pancreatic architecture, and are anemic, leukopenic, and thrombocytopenic. These phenotypes are accompanied by specific rather than wholesale changes in protein glycosylation, suggesting that while universally required, the functions of certain proteins and, as a consequence, certain cell types are especially sensitive to reductions in Pgm3 activity.
Figures
FIG. 1.
The Pgm3 mld1 and Pgm3 gt alleles of Pgm3 lead to aberrant mRNA splicing. (A) The Pgm3 mld1 allele contains a T-to-A transversion 14 bp upstream of exon 5. Electropherograms show the genomic DNA sequences from mice of the indicated genotypes. (B) The Pgm3 mld1 allele leads to aberrant mRNA splicing. RT-PCR was performed on splenic cDNA using primers located in exons 1 and 7 of Pgm3. An MslI recognition site is present in the 12-nt insertion; hence, PCR products were digested with MslI to distinguish correct splicing from splicing resulting in the 12-nt insertion. Size (bp) is displayed on the left. (C) Confirmation that the gene trap vector splices into Pgm3 mRNA. cDNA was prepared from W037B08 ES cells, and PCR was performed with the 5′ primer in exon 1 of Pgm3 and the 3′ primer in the gene trap vector. The no-reverse-transcriptase (−RT) control confirms that the PCR product is amplified from mRNA rather than from genomic DNA. (D) Schematic of aberrant mRNA splicing of the Pgm3 mld1 and Pgm3 gt alleles. Pale green, untranslated region; light blue, domain I; pink, domain II; dark green, domain III; purple, domain IV; dark blue, gene trap insertion; yellow, 12-nt insertion; black, poly(A) site. (E) Mutant proteins encoded by the Pgm3 mld1 and Pgm3 gt alleles. Light blue, domain I; pink, domain II; dark green, domain III; purple, domain IV; dark blue, gene trap insertion; yellow, 12-nt insertion.
FIG. 2.
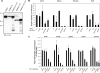
Proteins encoded by hypomorphic (Pgm3 mld1) and null (Pgm3 gt) alleles of Pgm3 have reduced enzymatic activity and result in decreased levels of UDP-GlcNAc in vivo. (A) Expression and purification of His-tagged Pgm3. Low yields were obtained of the two mutant proteins, Pgm3(+4) and Pgm3(Δ110); lanes with an asterisk (*) contain an approximately 10-fold concentration of the appropriate protein. Molecular mass (kDa) is displayed on the left. WB, Western blot. (B) Pgm3 enzyme assay on tissue extracts. Values represent the means ± standard errors of two tissue samples. (C) UDP-GlcNAc concentration in tissue extracts. Values represent the means ± standard errors of 3 to 11 tissue samples.
FIG. 3.
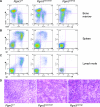
Mice with hypomorphic alleles of Pgm3 exhibit a profound B lymphopenia associated with a defect at the pro-B-cell-to-pre-B-cell transition. (A) Bone marrow cells from 60- to 80-day-old Pgm3+/+, Pgm3 mld1/mld1, and Pgm3 mld1/gt mice were analyzed by FACS for the expression of B220 and CD43. Data shown are representative of data from at least five age-matched mice. (B) Splenocytes from 60- to 80-day-old Pgm3+/+, Pgm3 mld1/mld1, and Pgm3 mld1/gt mice were analyzed by FACS for the expression of B220 and IgM. Data shown are representative of data from at least five age-matched mice. (C) Lymph node cells from 60- to 80-day-old Pgm3+/+, Pgm3 mld1/mld1, and Pgm3 mld1/gt mice were analyzed by FACS for expression of IgD and IgM. Data shown are representative of data from at least five age-matched mice. (D) Spleens of Pgm3 mld1/mld1 and Pgm3 mld1/gt mice display expansion of the red pulp and an absence of lymphoid follicles.
FIG. 4.
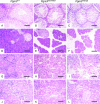
Mice with reduced Pgm3 enzyme activity and UDP-GlcNAc levels exhibit defective spermatogenesis, disrupted pancreatic and salivary gland architecture, and glomerulonephritis. Sections were stained with hematoxylin and eosin; the bar represents 60 μm. (A to C) Histopathology of testes. The testes of Pgm3 mld1/mld1 (B) and Pgm3 mld1/gt (C) mice contain immature spermatogonia, but all mature spermatozoa are pyknotic. (D to F) Histopathology of pancreas. In the pancreas of Pgm3 mld1/mld1 (E) and Pgm3 mld1/gt (F) mice, acini are reduced in size and dispersed. (G to I) Histopathology of salivary gland. In the salivary gland of Pgm3 mld1/mld1 (H) and Pgm3 mld1/gt (I) mice, cells are reduced in size and dispersed, with a lesion similar to that observed in the pancreas. (J to L) Kidneys of Pgm3 mld1/gt (L) but not Pgm3 mld1/mld1 (K) mice display glomerulonephritis with amorphous material filling the glomeruli.
FIG. 5.
Specific defects in glycosylation resulting from reductions in UDP-GlcNAc levels. (A and B) Membrane lysates were prepared from various tissues, separated by SDS-PAGE, and subjected to lectin blotting with P. vulgaris lectin (PHA-L) (A) or GS-II (B). Most glycoproteins were relatively unaffected by the reduced UDP-GlcNAc levels in Pgm3 mld1/mld1 mice; an exception was a ∼110-kDa glycoprotein in the testes (arrow). (C) Affinity purification of a ∼110-kDa glycoprotein from the testes of Pgm3+/+ but not Pgm3 mld1/mld1 mice. Membrane lysates from testes were subjected to affinity purification using GS-II-conjugated agarose. Eluted proteins were separated on an SDS-PAGE gel and visualized by Coomassie blue staining. The indicated band (arrow) was excised from the gel and identified by mass spectrometry as testicular ACE. (D) Lysates from Pgm3+/+ and Pgm3 mld1/mld1 testes were immunoprecipitated (IP) with an anti-ACE antibody that recognizes both somatic and testicular isoforms of ACE. Immunoprecipitates were separated by SDS-PAGE and subjected to lectin blotting with GS-II. The arrow indicates a band at the expected size of ACE, which is hypoglycosylated in Pgm3 mutants. (E) The blot in D was stripped and reprobed with anti-ACE antibody to demonstrate that testicular ACE is expressed in the Pgm3 mld1/mld1 testes but aberrantly glycosylated. (F) Red cell ghosts were prepared, and proteins were separated by SDS-PAGE. Coomassie blue staining demonstrated that major erythrocyte membrane proteins, including glycoproteins such as band 3, have normal molecular weights in Pgm3 mld1/mld1 red blood cells. (G) Red cell ghost preparations from F were subjected to Western blotting (WB) with anti-_O_-GlcNAc antibody. One band (*) is present in Pgm3+/+ and Pgm3 mld1/+ but absent in Pgm3 mld1/mld1 samples; another band (^) is present in Pgm3 mld1/+ and Pgm3 mld1/mld1 samples but absent in Pgm3+/+ samples. (H) Lysates were prepared, separated by SDS-PAGE, and subjected to Western blotting with anti-_O_-GlcNAc antibody.
FIG. 6.
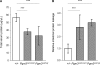
Intestinal protein leakage in Pgm3 mutant mice. (A) Total serum protein concentration measured by Bradford assay. (B) Levels of degradation-resistant AAT in fecal samples were measured by enzyme-linked immunosorbent assay. Data show relative levels between genotype classes, indicating intestinal protein leakage in Pgm3 mutants (***, P < 0.001).
Similar articles
- Genetic defects in the hexosamine and sialic acid biosynthesis pathway.
Willems AP, van Engelen BG, Lefeber DJ. Willems AP, et al. Biochim Biophys Acta. 2016 Aug;1860(8):1640-54. doi: 10.1016/j.bbagen.2015.12.017. Epub 2015 Dec 22. Biochim Biophys Acta. 2016. PMID: 26721333 Review. - A founder mutation underlies a severe form of phosphoglutamase 3 (PGM3) deficiency in Tunisian patients.
Ben-Khemis L, Mekki N, Ben-Mustapha I, Rouault K, Mellouli F, Khemiri M, Bejaoui M, Essaddam L, Ben-Becher S, Boughamoura L, Hassayoun S, Ben-Ali M, Barbouche MR. Ben-Khemis L, et al. Mol Immunol. 2017 Oct;90:57-63. doi: 10.1016/j.molimm.2017.06.248. Epub 2017 Jul 10. Mol Immunol. 2017. PMID: 28704707 - Hypomorphic homozygous mutations in phosphoglucomutase 3 (PGM3) impair immunity and increase serum IgE levels.
Sassi A, Lazaroski S, Wu G, Haslam SM, Fliegauf M, Mellouli F, Patiroglu T, Unal E, Ozdemir MA, Jouhadi Z, Khadir K, Ben-Khemis L, Ben-Ali M, Ben-Mustapha I, Borchani L, Pfeifer D, Jakob T, Khemiri M, Asplund AC, Gustafsson MO, Lundin KE, Falk-Sörqvist E, Moens LN, Gungor HE, Engelhardt KR, Dziadzio M, Stauss H, Fleckenstein B, Meier R, Prayitno K, Maul-Pavicic A, Schaffer S, Rakhmanov M, Henneke P, Kraus H, Eibel H, Kölsch U, Nadifi S, Nilsson M, Bejaoui M, Schäffer AA, Smith CI, Dell A, Barbouche MR, Grimbacher B. Sassi A, et al. J Allergy Clin Immunol. 2014 May;133(5):1410-9, 1419.e1-13. doi: 10.1016/j.jaci.2014.02.025. Epub 2014 Apr 1. J Allergy Clin Immunol. 2014. PMID: 24698316 Free PMC article. Clinical Trial. - Eleven percent intact PGM3 in a severely immunodeficient patient with a novel splice-site mutation, a case report.
Lundin KE, Wang Q, Hamasy A, Marits P, Uzunel M, Wirta V, Wikström AC, Fasth A, Ekwall O, Smith CIE. Lundin KE, et al. BMC Pediatr. 2018 Aug 29;18(1):285. doi: 10.1186/s12887-018-1258-9. BMC Pediatr. 2018. PMID: 30157810 Free PMC article. - Hyper-IgE syndromes: reviewing PGM3 deficiency.
Yang L, Fliegauf M, Grimbacher B. Yang L, et al. Curr Opin Pediatr. 2014 Dec;26(6):697-703. doi: 10.1097/MOP.0000000000000158. Curr Opin Pediatr. 2014. PMID: 25365149 Review.
Cited by
- Human hyper-IgE syndrome: singular or plural?
Zhang Q, Boisson B, Béziat V, Puel A, Casanova JL. Zhang Q, et al. Mamm Genome. 2018 Aug;29(7-8):603-617. doi: 10.1007/s00335-018-9767-2. Epub 2018 Aug 9. Mamm Genome. 2018. PMID: 30094507 Free PMC article. Review. - Congenital disorders of glycosylation: narration of a story through its patents.
Monticelli M, D'Onofrio T, Jaeken J, Morava E, Andreotti G, Cubellis MV. Monticelli M, et al. Orphanet J Rare Dis. 2023 Aug 29;18(1):247. doi: 10.1186/s13023-023-02852-w. Orphanet J Rare Dis. 2023. PMID: 37644541 Free PMC article. Review. - CDG Therapies: From Bench to Bedside.
Brasil S, Pascoal C, Francisco R, Marques-da-Silva D, Andreotti G, Videira PA, Morava E, Jaeken J, Dos Reis Ferreira V. Brasil S, et al. Int J Mol Sci. 2018 Apr 27;19(5):1304. doi: 10.3390/ijms19051304. Int J Mol Sci. 2018. PMID: 29702557 Free PMC article. Review. - Detection of phosphoglucomutase-3 deficiency by lectin-based flow cytometry.
Carlson RJ, Bond MR, Hutchins S, Brown Y, Wolfe LA, Lam C, Nelson C, DiMaggio T, Jones N, Rosenzweig SD, Stone KD, Freeman AF, Holland SM, Hanover JA, Milner JD, Lyons JJ. Carlson RJ, et al. J Allergy Clin Immunol. 2017 Jul;140(1):291-294.e4. doi: 10.1016/j.jaci.2016.12.951. Epub 2017 Jan 4. J Allergy Clin Immunol. 2017. PMID: 28063873 Free PMC article. No abstract available. - Dynamic O-GlcNAcylation and its roles in the cellular stress response and homeostasis.
Groves JA, Lee A, Yildirir G, Zachara NE. Groves JA, et al. Cell Stress Chaperones. 2013 Sep;18(5):535-58. doi: 10.1007/s12192-013-0426-y. Epub 2013 Apr 26. Cell Stress Chaperones. 2013. PMID: 23620203 Free PMC article.
References
- Alexander, W. S., A. W. Roberts, N. A. Nicola, R. Li, and D. Metcalf. 1996. Deficiencies in progenitor cells of multiple hematopoietic lineages and defective megakaryocytopoiesis in mice lacking the thrombopoietic receptor c-Mpl. Blood 87:2162-2170. - PubMed
- Ault, K. A., and C. Knowles. 1995. In vivo biotinylation demonstrates that reticulated platelets are the youngest platelets in circulation. Exp. Hematol. 23:996-1001. - PubMed
- Bigge, J. C., T. P. Patel, J. A. Bruce, P. N. Goulding, S. M. Charles, and R. B. Parekh. 1995. Nonselective and efficient fluorescent labeling of glycans using 2-amino benzamide and anthranilic acid. Anal. Biochem. 230:229-238. - PubMed
- Bode, L., and H. H. Freeze. 2006. Applied glycoproteomics—approaches to study genetic-environmental collisions causing protein-losing enteropathy. Biochim. Biophys. Acta 1760:547-559. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK055615/DK/NIDDK NIH HHS/United States
- R37 CA022556/CA/NCI NIH HHS/United States
- CA22556/CA/NCI NIH HHS/United States
- R01 CA022556/CA/NCI NIH HHS/United States
- R01 DK55615/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases