Huntingtin facilitates dynein/dynactin-mediated vesicle transport - PubMed (original) (raw)
Huntingtin facilitates dynein/dynactin-mediated vesicle transport
Juliane P Caviston et al. Proc Natl Acad Sci U S A. 2007.
Abstract
Cytoplasmic dynein is a multisubunit microtubule motor complex that, together with its activator, dynactin, drives vesicular cargo toward the minus ends of microtubules. Huntingtin (Htt) is a vesicle-associated protein found in both neuronal and nonneuronal cells that is thought to be involved in vesicular transport. In this study, we demonstrate through yeast two-hybrid and affinity chromatography assays that Htt and dynein intermediate chain interact directly; endogenous Htt and dynein co-immunoprecipitate from mouse brain cytosol. Htt RNAi in HeLa cells results in Golgi disruption, similar to the effects of compromising dynein/dynactin function. In vitro studies reveal that Htt and dynein are both present on vesicles purified from mouse brain. Antibodies to Htt inhibited vesicular transport along microtubules, suggesting that Htt facilitates dynein-mediated vesicle motility. In vivo inhibition of dynein function results in a significant redistribution of Htt to the cell periphery, suggesting that dynein transports Htt-associated vesicles toward the cell center. Together these findings indicate that Htt binds to dynein and acts in a complex along with dynactin and Htt-associated protein-1 to facilitate vesicular transport.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
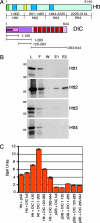
Mapping the interaction between Htt and DIC. (A) (Upper) Htt has a polyglutamine region (green), HEAT repeat clusters (dark blue), and DIC interaction domain (yellow). (Lower) DIC has an N-terminal-coiled domain (magenta), dynactin interaction domain (pink), Htt interaction domain (purple), and seven WD repeats (red). (B) Four peptides (Htt1–Htt4) spanning the length of Htt were _in vitro_-translated, loaded on a DIC column, and eluted with 2 M NaCl. The lanes are load (L), flow-through (F), wash (W), elution 1 (E1), and elution 2 (E2). Only Htt2 was eluted from the DIC column. (C) Four DIC truncations (1–120, 1–283, 120–283, and 283–644) were tested for interactions with Htt536−698 as assayed by β-galactosidase activity (SEM, n = 3). Controls include the activation domain (pAD) and DNA-binding domain (pDB) vectors.
Fig. 2.
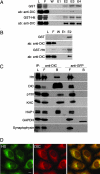
Htt interacts with endogenous cytoplasmic dynein. (A) Affinity chromatography of GST beads (Upper) or GST-Htt536−698 beads (Lower) incubated with mouse brain cytosol. SDS/PAGE gel samples from the load (L), flow-through (F), wash (W), and elutions (E1–E4) were stained to visualize GST or GST-Htt protein and then probed with a monoclonal anti-DIC antibody. DIC is present in fractions eluted with 20 μM glutathione from GST-Htt beads, but not from GST control beads. (B) Affinity chromatography of GST beads (Upper) or GST-Htt beads (Lower) incubated with FPLC-purified dynein derived from bovine brain. DIC is present in fraction E2 eluted from GST-Htt beads, but not from GST control beads. (C) Coimmunoprecipitation of endogenous Htt and DIC from mouse brain cytosol with a polyclonal antibody to DIC UP1467 (anti-DIC), but not a control antibody (anti-GFP). The load (L), flow-through (F), and bead (B) samples were probed with antibodies to Htt, DIC, dynactin (p150), kinesin heavy chain (KHC), and HAP1. Negative controls included GAPDH and synaptophysin. Blots probed for GAPDH showed some cross-reactivity with the GFP antibody light chain. (D) Htt and DIC have similar distributions in HeLa cells. Both Htt (green) and DIC (red) are distributed in tiny puncta throughout the cytoplasm, with overlapping concentrations at the Golgi in the perinuclear region (yellow). (Scale bar: 10 μm.)
Fig. 3.
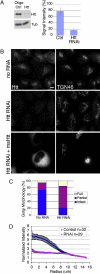
Htt RNAi causes disruption of Golgi. (A) (Left) HeLa cells were treated with Htt RNAi or control oligonucleotides; cell extracts were blotted with antibodies to Htt and tubulin. (Right) Htt RNAi decreased Htt expression to 16 ± 5% of no RNA controls, whereas negative control oligonucleotides only modestly decreased expression (SEM, n = 5). (B) (Top) No RNA control cells immunostained for Htt and TGN46 have a normal Golgi. (Middle) Htt immunostaining is absent in Htt RNAi cells and the Golgi is disorganized, with tubules stretched out into the cytoplasm. (Bottom) Htt RNAi cells were transfected with plasmid DNA encoding mouse Htt. The transfected cell in the field has a much brighter Htt signal than nontransfected cells, and Golgi disruption is rescued. (Scale bar: 10 μm.) (C) Golgi morphology in Htt RNAi and no RNA control cells immunostained for TGN46 was qualitatively assessed and binned into three categories: intact, partial (tubulated), and full disruption (vesicular) (SEM, n = 300). (D) Images of Htt RNAi (SEM, n = 29) and no RNA control cells (SEM, n = 32) immunostained for TGN46 were analyzed with a radial profiling algorithm that measured the signal intensity for a series of 110 concentric circles (normalized by dividing by the area) emanating from the center of each cell. Control cells (blue) had a normal Golgi morphology, and signal was concentrated near the cell center. In contrast, in Htt RNAi cells (pink), the TGN46 signal was delocalized from the cell center.
Fig. 4.
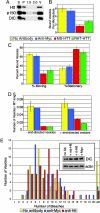
Functional Htt is required for dynein-mediated vesicle transport in vitro. (A) Htt copurifies in the vesicle fraction with dynein and dynactin. Vesicles purified from p50-GFP transgenic mouse brain were isolated by flotation through a sucrose step gradient. The high-speed supernatant (S), high-speed pellet (P), 1.5-M and 2.0-M steps of the gradient, as well as the 0.6- to 1.5-M interface containing the vesicles (V) were blotted with antibodies against Htt, p150Glued, and DIC. (B) Vesicles were preincubated with motility assay buffer containing no antibody (yellow), anti-Myc (blue), mouse monoclonal anti-Htt (red), or rat monoclonal anti-Htt (green) and then flowed into a chamber containing microtubules and analyzed by total internal reflection microscopy. Microtubules of similar lengths were analyzed (SEM, n = 15 for each condition). (C) Vesicle movement was reduced from 62 ± 4% in the presence of control antibody (SEM, n = 30) to 23 ± 7% in the presence of mouse monoclonal anti-Htt (SEM, n = 22). Stationary vesicles increased from 38 ± 4% in the presence of control antibody to 77 ± 7% in the presence of mouse monoclonal anti-Htt. (D) Inhibition of vesicle motility was bidirectional in assays repeated with polarity-marked microtubules. In the presence of mouse monoclonal anti-Htt, vesicle motility in both the minus-end and plus-end directions was reduced. (E) Dynein is not lost from vesicles in the presence of anti-Htt antibodies. Vesicles isolated as in A were preincubated with antibodies and then laser-bleached to quench the fluorescent signal of the p50-GFP; the number of bleaching events required to completely quench each vesicle was recorded. There were no significant differences between vesicles preincubated with no antibody (n = 50) (yellow), anti-Myc control antibody (n = 61) (blue), or mouse monoclonal anti-Htt (n = 68) (red). Vesicles requiring >20 bleach events were considered aggregates and excluded from further analysis. (Inset) Vesicles were preincubated with antibodies and then centrifuged at high speed. Pellets from vesicles preincubated with no antibody (no Ab), mouse monoclonal anti-Htt (ms-Htt), rat monoclonal anti-Htt (rt-Htt), or anti-Myc antibodies were blotted for DIC and actin (as a loading control).
Fig. 5.
Htt localization depends on functional dynein. (A) (Left) No RNA control cells immunostained for DIC and Htt as noted. (Right) DHC RNAi cells lack DIC, and Htt is redistributed to the periphery of cells (yellow arrowheads). (Scale bar: 10 μm.) (B) HeLa cells transfected with p50-GFP (green) have a disrupted Golgi (TGN46, blue), and Htt (red) is redistributed to the periphery of cells (white arrowheads). (Scale bar: 10 μm.) (C) Cortical Htt in DHC RNAi cells overlaps with microtubules (MT) (Upper) and F-actin (Lower). (Scale bar: 12 μm.) The boxed regions in the merged images are shown at a higher magnification in the far right panel. (Scale bar: 3 μm.) (D) Htt is at the center of a multipartite complex facilitating vesicle (gray) recruitment to microtubule motors involving dynein (light blue), dynactin (dark blue), Htt (yellow), HAP1 (red), and kinesin (purple). Arrow denotes direction of movement along microtubule (green).
Similar articles
- pARIS-htt: an optimised expression platform to study huntingtin reveals functional domains required for vesicular trafficking.
Pardo R, Molina-Calavita M, Poizat G, Keryer G, Humbert S, Saudou F. Pardo R, et al. Mol Brain. 2010 Jun 1;3:17. doi: 10.1186/1756-6606-3-17. Mol Brain. 2010. PMID: 20515468 Free PMC article. - Disruption of the dynein-dynactin complex unveils motor-specific functions in osteoclast formation and bone resorption.
Ng PY, Cheng TS, Zhao H, Ye S, Sm Ang E, Khor EC, Feng HT, Xu J, Zheng MH, Pavlos NJ. Ng PY, et al. J Bone Miner Res. 2013 Jan;28(1):119-34. doi: 10.1002/jbmr.1725. J Bone Miner Res. 2013. PMID: 22887640 - Chlamydia trachomatis uses host cell dynein to traffic to the microtubule-organizing center in a p50 dynamitin-independent process.
Grieshaber SS, Grieshaber NA, Hackstadt T. Grieshaber SS, et al. J Cell Sci. 2003 Sep 15;116(Pt 18):3793-802. doi: 10.1242/jcs.00695. Epub 2003 Aug 5. J Cell Sci. 2003. PMID: 12902405 - Regulation of dynein-dynactin-driven vesicular transport.
Liu JJ. Liu JJ. Traffic. 2017 Jun;18(6):336-347. doi: 10.1111/tra.12475. Epub 2017 Mar 28. Traffic. 2017. PMID: 28248450 Review. - [Dynein and dynactin as organizers of the system of cell microtubules].
Burakov AV, Nadezhdina ES. Burakov AV, et al. Ontogenez. 2006 Sep-Oct;37(5):323-39. Ontogenez. 2006. PMID: 17066975 Review. Russian.
Cited by
- Do Changes in Synaptic Autophagy Underlie the Cognitive Impairments in Huntington's Disease?
Grosso Jasutkar H, Yamamoto A. Grosso Jasutkar H, et al. J Huntingtons Dis. 2021;10(2):227-238. doi: 10.3233/JHD-200466. J Huntingtons Dis. 2021. PMID: 33780373 Free PMC article. Review. - The Huntington disease protein accelerates breast tumour development and metastasis through ErbB2/HER2 signalling.
Moreira Sousa C, McGuire JR, Thion MS, Gentien D, de la Grange P, Tezenas du Montcel S, Vincent-Salomon A, Durr A, Humbert S. Moreira Sousa C, et al. EMBO Mol Med. 2013 Feb;5(2):309-25. doi: 10.1002/emmm.201201546. Epub 2013 Jan 9. EMBO Mol Med. 2013. PMID: 23300147 Free PMC article. - HTT (huntingtin) and RAB7 co-migrate retrogradely on a signaling LAMP1-containing late endosome during axonal injury.
Krzystek TJ, White JA, Rathnayake R, Thurston L, Hoffmar-Glennon H, Li Y, Gunawardena S. Krzystek TJ, et al. Autophagy. 2023 Apr;19(4):1199-1220. doi: 10.1080/15548627.2022.2119351. Epub 2022 Sep 9. Autophagy. 2023. PMID: 36048753 Free PMC article. - Genetic and pharmacological inhibition of calcineurin corrects the BDNF transport defect in Huntington's disease.
Pineda JR, Pardo R, Zala D, Yu H, Humbert S, Saudou F. Pineda JR, et al. Mol Brain. 2009 Oct 27;2:33. doi: 10.1186/1756-6606-2-33. Mol Brain. 2009. PMID: 19860865 Free PMC article. - Striatal Vulnerability in Huntington's Disease: Neuroprotection Versus Neurotoxicity.
Morigaki R, Goto S. Morigaki R, et al. Brain Sci. 2017 Jun 7;7(6):63. doi: 10.3390/brainsci7060063. Brain Sci. 2017. PMID: 28590448 Free PMC article. Review.
References
- Caviston JP, Holzbaur EL. Trends Cell Biol. 2006;16:530–537. - PubMed
- DiFiglia M, Sapp E, Chase K, Schwarz C, Meloni A, Young C, Martin E, Vonsattel JP, Carraway R, Reeves SA. Neuron. 1995;14:1075–1081. - PubMed
- Hoffner G, Kahlem P, Djian P. J Cell Sci. 2002;115:941–948. - PubMed
- Block-Galarza J, Chase KO, Sapp E, Vaughn KT, Vallee RB, DiFiglia M, Aronin N. NeuroReport. 1997;8:2247–2251. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials