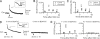Rapid bulk endocytosis and its kinetics of fission pore closure at a central synapse - PubMed (original) (raw)
Rapid bulk endocytosis and its kinetics of fission pore closure at a central synapse
Wei Wu et al. Proc Natl Acad Sci U S A. 2007.
Abstract
Upon exocytosis, fused vesicles must be retrieved for recycling. One route of retrieval is to generate endosome-like structures, from which small vesicles bud off. Endosome-like structures are widely thought to be generated slowly ( approximately 1 min) from the plasma membrane, a process called bulk endocytosis. Although the concept of bulk endocytosis seems established, the kinetic evidence showing the instant of the bulk membrane fission at synapses is still missing. The present work provides this missing piece of evidence at a calyx-type synapse. We used the capacitance measurement technique, which offers a high time resolution ( approximately 1 ms) to resolve the fission process. The instant of bulk membrane fission was reflected as a brief downward capacitance shift (DCS) of approximately 20-500 fF (mean = 123 fF) with 10-90% decay time of approximately 30-500 ms. At least 8.6-11.0% of exocytosed vesicles were retrieved by DCSs. During a DCS, the decrease in the fission pore conductance was detected, from which we found that the fission pore diameter decreased at approximately 39 nm/s. This provided the measurement of the rate of fission during bulk endocytosis at synapses. The DCS frequency peaked (approximately equal to 0.021 Hz) in <10 s after stimulation and decayed with a half time <20 s, indicating that the time course of bulk endocytosis is much faster than previously estimated with low time-resolution techniques. Our results also suggest that bulk endocytosis was composed of two kinetically different steps: the DCS that reflected the fission process and the time between stimulation and the DCS, during which membrane invagination led to the fission pore formation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
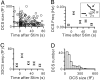
Fig. 1.
DCS reflects fission during bulk endocytosis. (A) Ten depolarizing pulses of 20 ms from −80 to +10 mV at 10 Hz (vertical arrow) induced a capacitance jump (Cm), which decayed smoothly most of time, except for an abrupt DCS (arrow). The DCS was readily identified when the capacitance decay was low-pass-filtered at 30 Hz (gray) and differentiated (Δ_Cm_/Δt; see
SI Text I
). Measured _G_m and _G_s are also shown. (B Left) Plot of the DCS in A in larger scales (same order as in A). The dashed line is a linear regression fit of the baseline before the DCS. (Center) A baseline-corrected, filtered Cm trace (Upper) was obtained by subtracting the dashed line from the low-pass-filtered capacitance trace (gray, Left). As described in
SI Text I
, this trace (Top) was used to predict changes in _G_m (Middle, red) and _G_s (Bottom, red). (Right) The measured (black) and the predicted (red) _G_m and _G_s shown in Left and Middle are superimposed. (C) Cm, _G_m, and _G_s traces averaged from 62 DCSs, each of which was >100 fF. The Cm trace was baseline-corrected (black), which was further low-pass-filtered at 30 Hz (gray) and used to predict changes in _G_m and _G_s (red, applies to C–E). (Scale bars apply to C–E.) (D) Similar to C, but a single DCS <100 fF. (E) Similar to C, but an average from 79 DCSs, each of which was <100 fF. (F) Equivalent circuit of the calyx without (Left) and with (Center and Right) bulk endocytosis. Labels are explained in the text.
Fig. 2.
The timing and the size of DCS. (A) DCS size plotted vs. the onset of DCSs (n = 141 DCSs). The stimulus (10 pulses of 20-ms depolarization at 10 Hz) was applied at time 0 (applies to Figs. 2–4). The stimulus was repeated one to four times in a calyx. A total of 154 stimuli were applied to 64 calyces. All data were superimposed. Analysis of these data gave rise to B–D. (B and C) The frequency (B) or accumulated amplitude of DCSs (ΣDCS amp, C) binned every 20 s per stimulus, is plotted vs. the time at which DCS occurred. Data are plotted as mean ± SE (applies to data with error bars in all figures). B Inset shows a DCS (large arrow) occurring at ≈3 s after the stimulus (vertical arrow). (D) DCS size distribution.
Fig. 3.
DCS frequency depends on the stimulus intensity and exocytosis. (A) A DCS occurred soon after a 20-ms depolarization. Inset enlarges the DCS. (B and C) The frequency (B) and the accumulated amplitude of DCSs (ΣDCS amp, C), per stimulus, binned every 20 s, are plotted vs. the DCS onset time after a 20-ms depolarization (triangles, n = 34 calyces). For comparison, the data after 10 pulses of 20-ms depolarization at 10 Hz are also plotted (gray circles, same as Fig. 2 B and C). (D) Sampled capacitance changes induced by 10 depolarizing pulses at 10 Hz at 3 min (Left) and 13 min (Right) after break in with a pipette containing BoNT/C (1 μM). D Inset shows DCSs in larger scales. (E) DCS frequency (per stimulus) plotted vs. the time after 10 pulses of 20-ms depolarization at 10 Hz. Data were obtained at 1–3 min (Left) and 10–15 min (Right) after break in with a pipette containing BoNT/C (1 μM) (n = 30 calyces).
Fig. 4.
Bulk endocytosis occurs after trains of AP-e. (A) Sampled capacitance changes and a DCS (arrow) induced by 500 AP-e at 100 Hz (vertical arrow). Inset shows the DCS in larger scales. (B and C) The frequency (B) and the accumulated amplitude (ΣDCS amp, C) of DCSs per stimulus, binned every 20 s, are plotted vs. the time after 500 AP-e at 100 Hz. The stimulus was applied one to four times at each of 46 calyces recorded. (D–F) Similar to A and B, but with a different stimulus (50 AP-e at 30 Hz, n = 52 calyces).
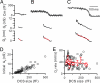
Fig. 5.
Fission _G_p measurements. (A–C) Baseline-corrected Cm, _G_p, and _D_p for three different sizes of DCSs (black). The 20–80% decrease of _D_p was fit with a linear regression line (red) with a slope (red) of −40 nm/s (A), −28 nm/s (B), and −31 nm/s (C), respectively. Cm trace was low-pass-filtered at 30 Hz (gray), from which _G_p and _D_p were calculated (Eqs. 10 and 11). (D) The detectable initial _G_p increased as the DCS size increased (circles). Data were obtained after 10 depolarizing pulses at 10 Hz (also applies to E). (E) The rate of _D_p closure during fission (_D_p rate) plotted vs. the DCS size (black circles). The data were also sorted based on the DCS size and averaged for every 10 data points. The resulting mean _D_p rate (±SE) was plotted vs. the corresponding mean DCS size (red). The red line is a linear regression fit of the mean data (correlation coefficient: −0.01).
Fig. 6.
Endocytosis is composed of two steps: membrane invagination and fission. (A) The number of DCS in the first 20 s after stimulation was not exhausted by repeated stimulation. The interval between the first and second stimulus (10 depolarizing pulses at 10 Hz) was 100 s (n = 15 calyces). Before these two stimuli, no stimulation was applied. (B) DCS onset time is independent of DCS size. DCS onset time (after 10 depolarizing pulses at 10 Hz) plotted vs. DCS size (open). The data were also sorted based on the DCS size and averaged for every 10 data points. The resulting mean onset time (±SE) was also plotted vs. the corresponding mean DCS size (red). The line is a linear regression fit of the mean data (correlation coefficient: −0.03). Data were taken from Fig. 2_A_. (C) A schematic drawing showing that endocytosis is composed of two steps, membrane invagination and fission.
Similar articles
- Synaptic vesicle recycling at the calyx of Held.
Xue L, Mei YA. Xue L, et al. Acta Pharmacol Sin. 2011 Mar;32(3):280-7. doi: 10.1038/aps.2010.212. Epub 2011 Jan 24. Acta Pharmacol Sin. 2011. PMID: 21258358 Free PMC article. Review. - Single and multiple vesicle fusion induce different rates of endocytosis at a central synapse.
Sun JY, Wu XS, Wu LG. Sun JY, et al. Nature. 2002 May 30;417(6888):555-9. doi: 10.1038/417555a. Nature. 2002. PMID: 12037569 - Multiple Modes of Fusion and Retrieval at the Calyx of Held Synapse.
Wu XS, Wu LG. Wu XS, et al. Adv Neurobiol. 2023;33:43-62. doi: 10.1007/978-3-031-34229-5_3. Adv Neurobiol. 2023. PMID: 37615863 - Exo-endocytosis and closing of the fission pore during endocytosis in single pituitary nerve terminals internally perfused with high calcium concentrations.
Rosenboom H, Lindau M. Rosenboom H, et al. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5267-71. doi: 10.1073/pnas.91.12.5267. Proc Natl Acad Sci U S A. 1994. PMID: 8202480 Free PMC article. - Bulk endocytosis at neuronal synapses.
Nguyen TH, Qiu X, Sun J, Meunier FA. Nguyen TH, et al. Sci China Life Sci. 2014 Apr;57(4):378-83. doi: 10.1007/s11427-014-4636-z. Epub 2014 Mar 18. Sci China Life Sci. 2014. PMID: 24643417 Review.
Cited by
- Exocytosis, Endocytosis, and Their Coupling in Excitable Cells.
Liang K, Wei L, Chen L. Liang K, et al. Front Mol Neurosci. 2017 Apr 19;10:109. doi: 10.3389/fnmol.2017.00109. eCollection 2017. Front Mol Neurosci. 2017. PMID: 28469555 Free PMC article. Review. - Post-fusion structural changes and their roles in exocytosis and endocytosis of dense-core vesicles.
Chiang HC, Shin W, Zhao WD, Hamid E, Sheng J, Baydyuk M, Wen PJ, Jin A, Momboisse F, Wu LG. Chiang HC, et al. Nat Commun. 2014;5:3356. doi: 10.1038/ncomms4356. Nat Commun. 2014. PMID: 24561832 Free PMC article. - Actin Is Crucial for All Kinetically Distinguishable Forms of Endocytosis at Synapses.
Wu XS, Lee SH, Sheng J, Zhang Z, Zhao WD, Wang D, Jin Y, Charnay P, Ervasti JM, Wu LG. Wu XS, et al. Neuron. 2016 Dec 7;92(5):1020-1035. doi: 10.1016/j.neuron.2016.10.014. Epub 2016 Nov 10. Neuron. 2016. PMID: 27840001 Free PMC article. - An acto-myosin II constricting ring initiates the fission of activity-dependent bulk endosomes in neurosecretory cells.
Gormal RS, Nguyen TH, Martin S, Papadopulos A, Meunier FA. Gormal RS, et al. J Neurosci. 2015 Jan 28;35(4):1380-9. doi: 10.1523/JNEUROSCI.3228-14.2015. J Neurosci. 2015. PMID: 25632116 Free PMC article. - Synaptic vesicle recycling at the calyx of Held.
Xue L, Mei YA. Xue L, et al. Acta Pharmacol Sin. 2011 Mar;32(3):280-7. doi: 10.1038/aps.2010.212. Epub 2011 Jan 24. Acta Pharmacol Sin. 2011. PMID: 21258358 Free PMC article. Review.
References
- Richards DA, Guatimosim C, Betz WJ. Neuron. 2000;27:551–559. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources