Inhibition of spinal microglial cathepsin S for the reversal of neuropathic pain - PubMed (original) (raw)
. 2007 Jun 19;104(25):10655-60.
doi: 10.1073/pnas.0610811104. Epub 2007 Jun 5.
Ping K Yip, John Grist, Clive Gentry, Amelia A Staniland, Fabien Marchand, Maliheh Dehvari, Glen Wotherspoon, Janet Winter, Jakir Ullah, Stuart Bevan, Marzia Malcangio
Affiliations
- PMID: 17551020
- PMCID: PMC1965568
- DOI: 10.1073/pnas.0610811104
Inhibition of spinal microglial cathepsin S for the reversal of neuropathic pain
Anna K Clark et al. Proc Natl Acad Sci U S A. 2007.
Abstract
A recent major conceptual advance has been the recognition of the importance of immune system-neuronal interactions in the modulation of brain function, one example of which is spinal pain processing in neuropathic states. Here, we report that in peripheral nerve-injured rats, the lysosomal cysteine protease cathepsin S (CatS) is critical for the maintenance of neuropathic pain and spinal microglia activation. After injury, CatS was exclusively expressed by activated microglia in the ipsilateral dorsal horn, where expression peaked at day 7, remaining high on day 14. Intrathecal delivery of an irreversible CatS inhibitor, morpholinurea-leucine-homophenylalanine-vinyl phenyl sulfone (LHVS), was antihyperalgesic and antiallodynic in neuropathic rats and attenuated spinal microglia activation. Consistent with a pronociceptive role of endogenous CatS, spinal intrathecal delivery of rat recombinant CatS (rrCatS) induced hyperalgesia and allodynia in naïve rats and activated p38 mitogen-activated protein kinase (MAPK) in spinal cord microglia. A bioinformatics approach revealed that the transmembrane chemokine fractalkine (FKN) is a potential substrate for CatS cleavage. We show that rrCatS incubation reduced the levels of cell-associated FKN in cultured sensory neurons and that a neutralizing antibody against FKN prevented both FKN- and CatS-induced allodynia, hyperalgesia, and p38 MAPK activation. Furthermore, rrCatS induced allodynia in wild-type but not CX3CR1-knockout mice. We suggest that under conditions of increased nociception, microglial CatS is responsible for the liberation of neuronal FKN, which stimulates p38 MAPK phosphorylation in microglia, thereby activating neurons via the release of pronociceptive mediators.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
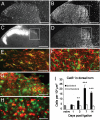
Fig. 1.
CatS is expressed by activated microglia in the dorsal horn of neuropathic rats. (A) CatS-ir is found in the dorsal horn of naïve rat lumbar spinal cord. (B) Increased CatS-ir in the lumbar dorsal horn, especially in the mediolateral part of the ipsilateral dorsal horn (outlined area) 14 days after PNL. (C) Lack of isolectin B4-ir in the mediolateral dorsal horn ipsilateral to PNL. (D) Ipsilateral OX42 staining. (E) Confocal image of CatS and OX42 in the dorsal horn. CatS (green) is coexpressed (yellow) with OX42 (red). (F–H) Confocal images of CatS (green) and GFAP (F, red), MAP2 (G, red), or NeuN (H, red). (Scale bars: 250 μm in A–D; 20 μm in E–G; and 10 μm in H.) (I) Temporal profile of CatS-ir in the dorsal horn after PNL. ∗∗, P < 0.01; ∗∗∗, P < 0.001 versus naïve tissues, four rats per group.
Fig. 2.
Spinal delivery of the CatS inhibitor LHVS is antinociceptive in neuropathic rats. Acute injection of LHVS (50 nmol per rat) is not antiallodynic in 3-day neuropathic rats (A) but is analgesic in 7-day (B) and 14-day (C) neuropathic rats. Intrathecal injection of LHVS (10 or 50 nmol per rat) reverses established neuropathic mechanical hyperalgesia in 14-day neuropathic rats (D). ∗, P < 0.05, ∗∗∗, P < 0.001 versus vehicle (Veh) group, six to eight rats per group. PWT, paw withdrawal threshold.
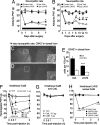
Fig. 3.
Prolonged spinal delivery of LHVS reverses tactile allodynia and attenuates microglia activation in neuropathic rats. rrCatS is pronociceptive in naïve rats. LHVS (30 nmol per rat per day) intrathecally delivered from day 0 to day 7 after injury (horizontal black bar) does not modify allodynia after 3 and 5 days but reverses allodynia after 7 days of treatment (A). LHVS delivered from day 7 to day 14 after injury (horizontal black bar) reverses established mechanical allodynia (B) and attenuates microglia activation (OX42-ir) (D) as compared with vehicle (C). (C and D) Ipsilateral dorsal horn (left) and contralateral dorsal horn (right) and high-power images (Insets: 20× magnification; scale bars: 50 μm) of the ipsilateral dorsal horn. (Scale bars: 100 μm.) (E) Quantitative analysis of OX42-ir. ∗∗∗, P < 0.001 versus vehicle group, four rats per group. (F) Intrathecal injection of activated rrCatS- (0.3–3 μg per rat) induced mechanical hypersensitivity in naïve rat hind paws, whereas nonactivated rrCatS (1 μg per rat) is ineffective. (G) Intrathecal activated CatB and CatL do not alter mechanical thresholds. (H) Intrathecal LHVS (50 nmol per rat, 30 min before CatS) prevents the hyperalgesia evoked by intrathecal activated rrCatS (1 μg per rat). ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001 versus vehicle group in A, B, and F; versus LHVS + rrCatS group in H, six to eight rats per group.
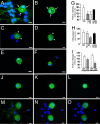
Fig. 4.
CatS incubation reduces the levels of sensory neuron-associated FKN. (A and B) DRG neurons in culture express FKN immunoreactivity (FKN-ir) (green) on the cell membrane (arrows) as well as intracellularly (asterisks). (C and D) Incubation with rrCatS (10 nM) for 30 min reduces FKN-ir (green) (arrowheads). (E and F) Inactivated (heat-denatured) CatS (10 nM) does not significantly reduce FKN-ir (green). (G) Quantitative analysis of FKN-ir in cultured DRG, three independent experiments. ∗∗, P < 0.01 versus vehicle. (H) Quantitative analysis of FKN protein in cultured DRG extracts by using ELISA. Activated rrCatS was incubated for 30 min. Data represent three independent experiments (two rats per experiment). ∗, P < 0.05 versus vehicle. (I) Quantitative analysis of membrane and intracellular FKN-ir at the midplane of cultured DRG (e.g., K and N). Data represent three independent experiments. ∗, P < 0.05. (J–O) A series of _z_-stack images of DRG neurons incubated without rrCatS (J–L) or in the presence of activated rrCatS (M–O). Images were taken with the Zeiss Axioplan 2 fluorescence microscope via a Zeiss ApoTome system. DAPI (blue) was used as a nuclear marker for all of the panels. (Scale bars: 10 μm.) I-CatS, inactivated CatS.
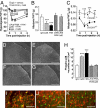
Fig. 5.
FKN-neutralizing antibody prevents CatS-induced hyperalgesia, allodynia, and activation of p38 MAPK in the dorsal horn. CatS-induced allodynia does not develop in CX3CR1-knockout mice. (A) Intrathecal injection of a rat FKN-neutralizing antibody (anti-FKN, 1 μg per rat) 1 h before intrathecal administration of either activated rrCatS (1 μg per rat) or FKN (amino acids 25–100; 30 ng per rat) prevents both CatS- and FKN-induced mechanical hyperalgesia. One microgram of IgG per rat was used as a control. ∗∗∗, P < 0.001 versus IgG + FKN or IgG + CatS. (B) Intrathecal injection of anti-FKN (1 μg per rat) 1 h before intrathecal administration of activated rrCatS (1 μg per rat, twice at 4-h intervals) or FKN (amino acids 25–100; 30 ng per rat) prevents CatS- and FKN-induced mechanical allodynia measured 1 h after CatS or FKN injections. ∗∗∗, P < 0.001 versus relevant control group (CatS, FKN, or Control IgG), six rats per group. (C) Intrathecal rrCatS (1.5 μg per mouse, twice at 4-h intervals) induces allodynia in wild-type but not CX3CR1-knockout mice. ∗, P < 0.05 versus wild-type mouse values at time 0. ∗∗, P < 0.01; ∗∗∗, P < 0.001 versus wild-type mice, six mice per group. (D–G) Activation of p38 MAPK after intrathecal delivery of control IgG (1 μg per rat) (D), FKN (30 ng per rat) (E), or activated rrCatS (1 μg per rat) (F) 15 min after injection. Anti-FKN (1 μg per rat) prevented rrCatS-induced p38 activation (G). (Scale bars: D–G, 100 μm.) (H) Quantitative analysis of p38 phosphorylation in the dorsal horn. ∗∗∗, P < 0.001, four rats per group. (I) CatS-activated p38 (green) colocalizes with OX42 (red) in the dorsal horn, but not with either GFAP (J, red) or NeuN (K, red). (Scale bars: I–K, 50 μm.)
Similar articles
- The liberation of fractalkine in the dorsal horn requires microglial cathepsin S.
Clark AK, Yip PK, Malcangio M. Clark AK, et al. J Neurosci. 2009 May 27;29(21):6945-54. doi: 10.1523/JNEUROSCI.0828-09.2009. J Neurosci. 2009. PMID: 19474321 Free PMC article. - Role of the CX3CR1/p38 MAPK pathway in spinal microglia for the development of neuropathic pain following nerve injury-induced cleavage of fractalkine.
Zhuang ZY, Kawasaki Y, Tan PH, Wen YR, Huang J, Ji RR. Zhuang ZY, et al. Brain Behav Immun. 2007 Jul;21(5):642-51. doi: 10.1016/j.bbi.2006.11.003. Epub 2006 Dec 15. Brain Behav Immun. 2007. PMID: 17174525 Free PMC article. - Microglial signalling mechanisms: Cathepsin S and Fractalkine.
Clark AK, Malcangio M. Clark AK, et al. Exp Neurol. 2012 Apr;234(2):283-92. doi: 10.1016/j.expneurol.2011.09.012. Epub 2011 Sep 17. Exp Neurol. 2012. PMID: 21946268 Review. - Role of the immune system in neuropathic pain.
Malcangio M. Malcangio M. Scand J Pain. 2019 Dec 18;20(1):33-37. doi: 10.1515/sjpain-2019-0138. Scand J Pain. 2019. PMID: 31730538 Review.
Cited by
- Redefining the concept of protease-activated receptors: cathepsin S evokes itch via activation of Mrgprs.
Reddy VB, Sun S, Azimi E, Elmariah SB, Dong X, Lerner EA. Reddy VB, et al. Nat Commun. 2015 Jul 28;6:7864. doi: 10.1038/ncomms8864. Nat Commun. 2015. PMID: 26216096 Free PMC article. - Concentration and proteolysis of CX3CL1 may regulate the microglial response to CX3CL1.
Finneran D, Li Q, Subbarayan MS, Joly-Amado A, Kamath S, Dengler DG, Gordon MN, Jackson MR, Morgan D, Bickford PC, Smith LH, Nash KR. Finneran D, et al. Glia. 2023 Feb;71(2):245-258. doi: 10.1002/glia.24269. Epub 2022 Sep 15. Glia. 2023. PMID: 36106533 Free PMC article. - Cellular and molecular mechanisms of pain.
Basbaum AI, Bautista DM, Scherrer G, Julius D. Basbaum AI, et al. Cell. 2009 Oct 16;139(2):267-84. doi: 10.1016/j.cell.2009.09.028. Cell. 2009. PMID: 19837031 Free PMC article. Review. - A state-of-the-art perspective on microgliopathic pain.
Inoue K. Inoue K. Open Biol. 2018 Nov 28;8(11):180154. doi: 10.1098/rsob.180154. Open Biol. 2018. PMID: 30487300 Free PMC article. Review. - Neuroimmune Mechanisms Underlying Neuropathic Pain: The Potential Role of TNF-α-Necroptosis Pathway.
Duan YW, Chen SX, Li QY, Zang Y. Duan YW, et al. Int J Mol Sci. 2022 Jun 28;23(13):7191. doi: 10.3390/ijms23137191. Int J Mol Sci. 2022. PMID: 35806192 Free PMC article. Review.
References
- Scholz J, Woolf CJ. Nat Neurosci. 2002;5:1062–1067. - PubMed
- Gilron I, Max MB. Exp Rev Neurother. 2005;5:823–830. - PubMed
- Watkins LR, Maier SF. Nat Rev Drug Discovery. 2003;2:973–985. - PubMed
- Tsuda M, Inoue K, Salter MW. Trends Neurosci. 2005;28:101–107. - PubMed
- Barclay J, Clark A, Ganju P, Gentry C, Patel S, Wotherspoon G, Buxton F, Chuanzheng S, Ullah J, Winter J, et al. Pain. 2007 in press. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous