Glucose phosphorylation is required for insulin-dependent mTOR signalling in the heart - PubMed (original) (raw)
Glucose phosphorylation is required for insulin-dependent mTOR signalling in the heart
Saumya Sharma et al. Cardiovasc Res. 2007.
Abstract
Objective: Insulin regulates both glucose uptake and postnatal cardiac growth. The anabolic effects of insulin are mediated by the mammalian target of rapamycin (mTOR), an evolutionarily conserved kinase which is also a convergence point between nutrient sensing and cell growth. We postulated that mTOR signalling in the heart requires the metabolism of glucose.
Methods: We interrogated the insulin-mediated mTOR signalling pathway in response to different metabolic interventions regulating substrate metabolism in the isolated working rat heart and in isolated cardiomyocytes.
Results: Although insulin enhanced Akt activity, phosphorylation of mTOR and its downstream targets (p70S6K and 4EBP1) required the addition of glucose. Glucose-dependent p70S6K phosphorylation was independent of the hexosamine biosynthetic pathway, the AMP kinase pathway, and the pentose phosphate pathway. However, inhibition of glycolysis downstream of hexokinase markedly enhanced p70S6K phosphorylation. Furthermore, 2-deoxyglucose activated p70S6K suggesting that phosphorylation of glucose is required for carbohydrate-mediated mTOR signalling in the heart. Lastly, we also found enhanced p70S6K phosphorylation in the hearts of diabetic rats.
Conclusion: Phosphorylation of glucose is necessary for insulin-dependent mTOR activity in the heart, suggesting a link between intermediary metabolism and cardiac growth.
Figures
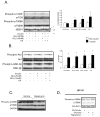
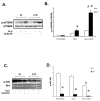
Figure 1. Immunoblots and densitometry ratios
A. Immunoblots (n=5) of mTOR signalling proteins in isolated working rat hearts perfused glucose (GLU) at 5mM and 15mM, and amino acids (AA) at physiologic plasma concentrations. Densitometry of the ratio of phosphorylated and total p70S6K (n=5). B. Immunoblots (n=5) of phosphorylated and total Akt and GSK 3β in isolated working rat hearts perfused, with GLU (5mM and 15mM), and AA. C. p70S6K signalling in working rat hearts perfused GLU (5mM) after animals were gavaged for 7 days with either vehicle or rapamycin. D. Immunoblot (n=3) of p70S6K signalling in neonatal cardiomyocytes incubated in PBS and insulin (1μg/ml) ± rapamycin (0.1μM), with the addition of glucose (5mM).* p< 0.05 compared to no insulin
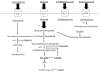
Figure 2. Ratoinale for the experimental strategy
Glucose is taken up by glucose transporters (GLUT) and phosphorylated inside the myocyte by hexokinase. Glucose 6-phosphate may and enter a number of pathways. Glycolysis and glucose oxidation result in ATP generation which may inhibit AMP kinase. Fructose 6-phosphate is converted to glucosamine-6-phosphate and enters the hexosamine biosynthetic pathway ultimately leading to O-GlcNacylation of proteins. Glucose 6-phosphate can also enter the pentose phosphate pathway and generate xylulose-5-phophate. In our experiments we utilized several substrates, activators, or inhibitors to assess whether glucose 6-phosphate regulates mTOR activation. These are underlined. Inhibitors or activators are in italics as well. AICAR 5-aminoimidazole-4-carboxamide ribonucleotide. GFAT glutamine:fructose 6-phosphate amidotransferase, HK hexokinase.
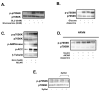
Figure 3. Immunoblots
A. Phosphorylation of p70S6K (n=3) in working rat hearts perfused with GLU (5mM), insulin (40μU/ml), Glucosamine (5mM). B. p70S6K signalling in rat hearts perfused (n=6) with GLU (5mM) + insulin (40μU/ml) with the addition of azaserine (10 mM and 20 mM). C. Immunoblot of p70S6K, AMP kinase, ACC phosphorylation in rat hearts perfused with GLU (5mM) + Insulin with the addition of AICAR (0.4mM). D. Immunoblot (n=3) of p70S6K phosphorylation in neonatal cardiomyocytes incubated in Ham's F-10 media + insulin (1μg/ml) and AICAR (1mM) or azaserine (10mM). E. p70S6K phosphorylation in rat hearts perfused with PLC/AcAc + Insulin (40μU/ml) with the addition of xylitol (0.5mM) (n=3).
Figure 4. Immunoblots
A. p70S6K phosphorylation in rat hearts perfused with insulin (40μU/ml) with the addition of pyruvate (0.05mM)/lactate (0.5mM) (n=3). B. Immunoblot (n=3) of p70S6K signalling in neonatal cardiomyocytes incubates in PBS or media ± insulin (1μg/ml) with the addition of AICAR (1mM) or iodoacetate (10mM). C. ACC phosphorylation in the cardiomyocytes exposed to AICAR and iodoacetate. D. Immunoblot (n=3) of p70S6K signalling in neonatal cardiomyocytes incubates in PBS+ insulin (1μg/ml) with the addition of GLU (5 mM and 15mM), 3-O methylglucose (3-O-MG) (5mM and 15mM), or 2-deoxyglucose (2-DG) (5mM and 15mM). E. Phosphorylation of p70S6K (n=3) in rat hearts perfused with PLC/AcAc + insulin (40μU/ml) with the addition of 2-DG (5mM).
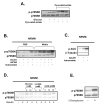
Figure 5. Immunoblots and densitometry ratios
A. p70S6K phosphorylation in ZDF rat hearts perfused with insulin (40μU/ml) with the addition of glucose (5 mM) alone or with glucose (5 mM) and sodium oleate (0.4mM) (n=3). B. Densitometry of the ratio of phosphorylated and total p70S6K (n=3) C. Akt phosphorylation in ZDF rat hearts perfused with insulin (40μU/ml) with the addition of glucose (5 mM) alone or with glucose (5 mM) and sodium oleate (0.4mM) (n=3). D. Densitometry of the ratio of phosphorylated and total Akt (n=3). * p<0.01 between ZDF and ZL rat hearts. # p<0.05 between GLU and GLU+OLE
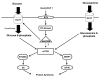
Figure 6. The proposed scheme for glucose 6-phosphate mediated mTOR signalling in the heart
Insulin activates PI3 kinase and Akt, which increases the uptake of glucose (by translocation of GLUT4 to the cell surface). Once it is phosphorylated, glucose induces mTOR activation. Glucosamine is phosphorylated to glucosamine 6-phosphate, which likely participates in mTOR signalling. Glycogen breakdown provides alternative carbohydrate source for mTOR activation. It is unknown whether Akt/PKB directly participates in mTOR signalling in the heart. Abbreviations: GLUT, glucose transporter; RTK, receptor tyrosine kinase; IGF1, insulin-like growth factor 1; PKB, protein kinase B; mTOR, mammalian target of rapamycin.
Similar articles
- Inhibition of the mTOR/p70S6K pathway is not involved in the insulin-sensitizing effect of AMPK on cardiac glucose uptake.
Ginion A, Auquier J, Benton CR, Mouton C, Vanoverschelde JL, Hue L, Horman S, Beauloye C, Bertrand L. Ginion A, et al. Am J Physiol Heart Circ Physiol. 2011 Aug;301(2):H469-77. doi: 10.1152/ajpheart.00986.2010. Epub 2011 May 20. Am J Physiol Heart Circ Physiol. 2011. PMID: 21602475 - Immunohistochemical analysis of the mammalian target of rapamycin signalling pathway in extramammary Paget's disease.
Chen S, Nakahara T, Uchi H, Takeuchi S, Takahara M, Kido M, Dugu L, Tu Y, Moroi Y, Furue M. Chen S, et al. Br J Dermatol. 2009 Aug;161(2):357-63. doi: 10.1111/j.1365-2133.2009.09179.x. Epub 2009 Apr 29. Br J Dermatol. 2009. PMID: 19438435 - Acetaldehyde promotes rapamycin-dependent activation of p70(S6K) and glucose uptake despite inhibition of Akt and mTOR in dopaminergic SH-SY5Y human neuroblastoma cells.
Fang CX, Yang X, Sreejayan N, Ren J. Fang CX, et al. Exp Neurol. 2007 Jan;203(1):196-204. doi: 10.1016/j.expneurol.2006.08.002. Epub 2006 Sep 7. Exp Neurol. 2007. PMID: 16962100 - Insulin resistance due to nutrient excess: is it a consequence of AMPK downregulation?
Saha AK, Xu XJ, Balon TW, Brandon A, Kraegen EW, Ruderman NB. Saha AK, et al. Cell Cycle. 2011 Oct 15;10(20):3447-51. doi: 10.4161/cc.10.20.17886. Cell Cycle. 2011. PMID: 22067655 Free PMC article. Review. - AKT signalling in the failing heart.
Chaanine AH, Hajjar RJ. Chaanine AH, et al. Eur J Heart Fail. 2011 Aug;13(8):825-9. doi: 10.1093/eurjhf/hfr080. Epub 2011 Jun 30. Eur J Heart Fail. 2011. PMID: 21724622 Free PMC article. Review.
Cited by
- Insulin signaling in the heart.
Abel ED. Abel ED. Am J Physiol Endocrinol Metab. 2021 Jul 1;321(1):E130-E145. doi: 10.1152/ajpendo.00158.2021. Epub 2021 May 31. Am J Physiol Endocrinol Metab. 2021. PMID: 34056923 Free PMC article. Review. - The two faces of miR-29.
Ślusarz A, Pulakat L. Ślusarz A, et al. J Cardiovasc Med (Hagerstown). 2015 Jul;16(7):480-90. doi: 10.2459/JCM.0000000000000246. J Cardiovasc Med (Hagerstown). 2015. PMID: 25689084 Free PMC article. Review. - Amino acids and derivatives, a new treatment of chronic heart failure?
Carubelli V, Castrini AI, Lazzarini V, Gheorghiade M, Metra M, Lombardi C. Carubelli V, et al. Heart Fail Rev. 2015 Jan;20(1):39-51. doi: 10.1007/s10741-014-9436-9. Heart Fail Rev. 2015. PMID: 24925377 Review. - Loss of long-chain acyl-CoA synthetase isoform 1 impairs cardiac autophagy and mitochondrial structure through mechanistic target of rapamycin complex 1 activation.
Grevengoed TJ, Cooper DE, Young PA, Ellis JM, Coleman RA. Grevengoed TJ, et al. FASEB J. 2015 Nov;29(11):4641-53. doi: 10.1096/fj.15-272732. Epub 2015 Jul 28. FASEB J. 2015. PMID: 26220174 Free PMC article. - Glucose regulation of load-induced mTOR signaling and ER stress in mammalian heart.
Sen S, Kundu BK, Wu HC, Hashmi SS, Guthrie P, Locke LW, Roy RJ, Matherne GP, Berr SS, Terwelp M, Scott B, Carranza S, Frazier OH, Glover DK, Dillmann WH, Gambello MJ, Entman ML, Taegtmeyer H. Sen S, et al. J Am Heart Assoc. 2013 May 17;2(3):e004796. doi: 10.1161/JAHA.113.004796. J Am Heart Assoc. 2013. PMID: 23686371 Free PMC article.
References
- Rohde J, Heitman J, Cardenas ME. The tor kinases link nutrient sensing to cell growth. J Biol Chem. 2001;276:9583–6. - PubMed
- Proud CG. Role of mtor signalling in the control of translation initiation and elongation by nutrients. Curr Top Microbiol Immunol. 2004;279:215–44. - PubMed
- Barbieri M, Bonafe M, Franceschi C, Paolisso G. Insulin/igf-i-signaling pathway: An evolutionarily conserved mechanism of longevity from yeast to humans. Am J Physiol Endocrinol Metab. 2003;285:E1064–71. - PubMed
- Moule SK, Denton RM. Multiple signaling pathways involved in the metabolic effects of insulin. Am J Cardiol. 1997;80:41A–49A. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 HL007591/HL/NHLBI NIH HHS/United States
- R01 HL043133/HL/NHLBI NIH HHS/United States
- 5T32 HL 07591/HL/NHLBI NIH HHS/United States
- R01 HL043133-12/HL/NHLBI NIH HHS/United States
- R01 HL 43133/HL/NHLBI NIH HHS/United States
- T32 HL007591-15/HL/NHLBI NIH HHS/United States
- R01 HL061483/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous