Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation - PubMed (original) (raw)
Comparative Study
Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation
Christopher G Vecsey et al. J Neurosci. 2007.
Abstract
Histone deacetylase (HDAC) inhibitors increase histone acetylation and enhance both memory and synaptic plasticity. The current model for the action of HDAC inhibitors assumes that they alter gene expression globally and thus affect memory processes in a nonspecific manner. Here, we show that the enhancement of hippocampus-dependent memory and hippocampal synaptic plasticity by HDAC inhibitors is mediated by the transcription factor cAMP response element-binding protein (CREB) and the recruitment of the transcriptional coactivator and histone acetyltransferase CREB-binding protein (CBP) via the CREB-binding domain of CBP. Furthermore, we show that the HDAC inhibitor trichostatin A does not globally alter gene expression but instead increases the expression of specific genes during memory consolidation. Our results suggest that HDAC inhibitors enhance memory processes by the activation of key genes regulated by the CREB:CBP transcriptional complex.
Figures
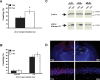
Figure 1.
Intrahippocampal injection of TSA enhances the consolidation of contextual fear memory and increases histone H3 acetylation in the hippocampus. A, C57BL/6J male mice were fitted with intrahippocampal cannulas, fear conditioned, and injected with either VEH or TSA (16.5 m
m
TSA). Mice receiving TSA immediately after conditioning (n = 11) exhibited a significant increase in levels of freezing in a 24 h retention test, performed in the same conditioned context compared with mice receiving vehicle (n = 11). B, Mice receiving either TSA (n = 5) or vehicle (n = 6) immediately after conditioning showed no difference in levels of freezing during a cued fear conditioning test 24 h after conditioning, either before (Pre CS) or during (CS) presentation of the tone cue. C, Western blot analysis of acid-extracted nuclear lysates shows increased histone H3 acetylation in the hippocampus taken from mice injected with TSA (16.5 m
m
TSA) compared with mice injected with vehicle. Samples were obtained 2, 4, or 24 h after injection and normalized by Bradford assay. Anti-β-actin signal shows equivalent loading of protein samples in each lane. D, Intrahippocampal injection of TSA locally increases histone H3 acetylation in the hippocampus. Perfusion-fixed and immunostained coronal brain sections were prepared from mice killed 4 h after receiving intrahippocampal injections of vehicle or TSA (16.5 m
m
TSA). A representative section from a mouse treated with vehicle shows negligible histone H3 acetylation (VEH, top right). Bottom right shows higher-magnification 40× image of CA1. A representative section from a mouse treated with TSA shows increased histone H3 acetylation in hippocampal area CA1 (TSA, top left). Bottom left shows higher-magnification 40× image of CA1. Nuclear staining with DAPI demonstrates normal hippocampal morphology in sections from both vehicle- and TSA-treated mice. DG, Dentate gyrus. *p < 0.05.
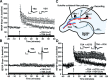
Figure 2.
TSA enhances one-train E-LTP via a transcription-dependent mechanism. A, Long-term potentiation (E-LTP) was induced by a single 1 s, 100 Hz train in the CA1 region of hippocampal slices from C57BL/6J mice in the presence of VEH or TSA (1.65 μ
m
). Slices treated with vehicle (n = 8 slices from 7 mice) showed a transient potentiation that decays to baseline by 60 min, whereas slices treated with TSA (n = 12 slices from 6 mice) showed a significantly more robust and longer-lasting potentiation (p < 0.05). Vehicle or TSA was administered throughout the experiment. **_B_**, There was no effect of TSA (_n_ = 12 slices from 6 mice) compared with vehicle (_n_ = 8 slices from 7 mice) on baseline responses in a second pathway that did not receive tetanization (_p_ > 0.05). Insets in A and B show superimposed sample sweeps from the first 5 and last 5 min of the recording for VEH- and TSA-treated slices. Calibration: 5 mV, 5 ms. C, Schematic diagram showing two-pathway LTP induction protocol (adapted from Kandel et al., 2000). Both pathways received alternating baseline stimulation every minute, but LTP was only elicited in one pathway, with the other serving as a control. D, Treatment with the transcription inhibitor ActD (25 μ
m
) blocks the enhancement of E-LTP by TSA. In this experiment, a single stimulating electrode was used, and solutions containing TSA+VEH or TSA+ActD were perfused for 20 min before tetanization and throughout the 2 h recording period. TSA+VEH-treated slices (n = 4 slices from 3 mice) have significantly enhanced LTP 100–120 min after tetanic stimulation (p < 0.05) compared with slices treated with TSA+ActD (n = 4 slices from 3 mice). Insets show superimposed average sample sweeps from the first 5 and last 5 min of the recording for TSA+VEH- and TSA+ActD-treated slices. Calibration: 5 mV, 5 ms.
Figure 3.
Enhancing histone acetylation by treatment with TSA does not enhance memory for contextual fear in CREBαΔ mutant mice. CREBαΔ mutant mice and wild-type (WT) littermates were fitted with intrahippocampal cannulas, subjected to contextual fear conditioning, and then immediately injected with either VEH or TSA (16.5 or 33 m
m
TSA). Injected mice were given a retrieval test 24 h later. Similar to a previous study examining contextual fear conditioning in CREBαΔ mutant mice (Graves et al., 2002), CREBαΔ mutant mice treated with vehicle (n = 6) showed significantly lower levels of freezing than wild-type littermates treated with vehicle (n = 6). Wild-type mice treated with either 16.5 m
m
TSA (n = 6) or 33 m
m
TSA (n = 6) showed significantly higher levels of freezing than wild-type mice treated with vehicle (n = 6), recapitulating results observed in C57BL/6J mice (Fig. 1). In contrast, no differences in levels of freezing were observed between CREBαΔ mutant mice treated with 16.5 m
m
TSA (n = 5) or 33 m
m
TSA (n = 6) and CREBαΔ mutant mice treated with vehicle (n = 6). *p < 0.05.
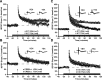
Figure 4.
Increasing histone acetylation by treatment with TSA does not enhance one-train E-LTP in CREBαΔ or _cbp_KIX/KIX mutant mice. LTP was induced by a single 1 s, 100 Hz train in the CA1 region of hippocampal slices in the presence of VEH or TSA (1.65 μ
m
). Hippocampal slices were treated with vehicle or TSA (indicated by black line) for 20 min before tetanization (indicated by an arrow) and throughout the recording period. A, Hippocampal slices from wild-type (WT) CREB+/+ mice treated with vehicle (n = 9 slices from 7 mice) showed a transient potentiation that gradually decreased to baseline. In contrast, slices treated with TSA (n = 9 slices from 9 mice) showed a significantly more robust and longer-lasting potentiation. Insets in A show superimposed sample sweeps from the first 5 and last 5 min of the recording for VEH- and TSA-treated slices. B, Hippocampal slices from CREBαΔ mutant mice treated with TSA (n = 9 slices from 9 mice) failed to produce robust and longer-lasting potentiation induced by one train paired with TSA compared with vehicle-treated slices (n = 11 slices from 9 mice). Insets in B show superimposed sample sweeps from the first 5 and last 5 min of the recording for VEH- and TSA-treated slices. Calibration: 5 mV, 5 ms. C, Hippocampal slices from wild-type cbp+/+ mice treated with TSA (n = 6 slices from 6 mice) showed a significant enhancement in E-LTP compared with VEH-treated slices (n = 5 slices from 5 mice). D, In contrast, hippocampal slices from _cbp_KIX/KIX homozygous mutant mice showed no enhancement in E-LTP induced in the presence of TSA (n = 7 slices from 5 mice) as opposed to VEH (n = 7 slices from 6 mice). Insets in C and D show averaged sample sweeps from the first 5 and last 5 min of the recording. Calibration: 5 mV, 5 ms.
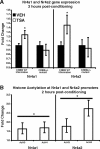
Figure 5.
TSA treatment after contextual fear conditioning affects the expression of only a subset of CREB target genes. A, CREBαΔ mutant mice and wild-type (WT) littermates were fitted with intrahippocampal cannulas, subjected to contextual fear conditioning, and then immediately injected with either vehicle (50% EtOH) or TSA (16.5 m
m
TSA). At 2 h after conditioning, mice were killed, hippocampi were removed, and total RNA was purified for conversion into cDNA. Expression levels were normalized to the following house keeping genes: Hprt, Actg, and Tubulin. There were no significant differences in gene expression for Nr4a1 and Nr4a2 in CREBαΔ mutant mice treated with TSA compared with those receiving vehicle. In contrast, the expression of Nr4a1 and Nr4a2 was significantly increased in CREB wild-type littermates treated with TSA. *p < 0.05. B, TSA treatment after contextual fear conditioning enhances histone acetylation at Nr4a1 and Nr4a2 promoters. Animals were cannulated and injected with TSA or vehicle. At 2 h after conditioning, mice were killed, and hippocampi were removed and crosslinked with formaldehyde. Chromatin was immunoprecipitated with anti-acetylated histone H3 and H4 antibodies (AcH3, AcH4). Immunoprecipitated DNA was quantified for enrichment of Nr4a1 and Nr4a2 promoter, normalized to input chromatin, and expressed as a ratio to vehicle-injected animals. *p < 0.05 for comparison of histone acetylation (H3 and H4) between drug- and vehicle-treated animals.
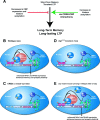
Figure 6.
HDAC inhibition enhances memory and synaptic plasticity via a CREB:CBP interaction. A, Decreases in CBP activity and histone acetylation impair long-term memory and long-lasting forms of LTP. Conversely, increasing histone acetylation enhances long-term memory and LTP (as shown in this study; for other references, see Discussion). Our results demonstrate that these enhancements occur via a CREB:CBP complex and transcription of particular CREB-regulated genes such as Nr4a1 and Nr4a2. B, A schematic diagram of how activation of CREB by phosphorylation in wild-type mice recruits CBP via the CREB-binding domain (KIX; shown as yellow crescent). CBP in turn becomes activated (possibly by phosphorylation via CaMKIV) (Impey et al., 2002) and then regulates CREB-mediated transcription by interacting with the basal transcription machinery and associated cofactors and via its intrinsic histone acetyltransferase activity. HDAC inhibition requires CREB:CBP interaction to effectively enhance memory and synaptic plasticity. C, In the absence of CREB, as in the CREBαΔ mutant mice, increasing histone acetylation via HDAC inhibition has no effect because there is no transcription factor present to recruit the transcriptional coactivator CBP, which further recruits basal transcription machinery and acetylates histones to facilitate gene expression. D, Similarly, in _cbp_KIX/KIX mice that express a mutant CBP protein that blocks the main interaction with CREB, HDAC inhibition is also ineffective. This demonstrates that the interaction between CREB and CBP is necessary for the enhancement of memory and synaptic plasticity by HDAC inhibition, presumably because CBP function cannot be compensated for by simply increasing histone acetylation via HDAC inhibitors. Thus, it is likely that the role CBP plays in recruiting basal transcription machinery or other factors is crucial for gene expression required for the enhancement of memory and synaptic plasticity by HDAC inhibition. E, In other Cbp mutant mice, including Cbp heterozygous mice (Alarcon et al., 2004), transgenic mice expressing a mutant CBP protein with a mutation in the HAT domain (Korzus et al., 2004), and transgenic mice expressing a truncated inhibitory form of CBP (Wood et al., 2005), there is still at least one wild-type allele of Cbp present. This wild-type allele is sufficient to support the enhancement of memory and synaptic plasticity by HDAC inhibitors, because wild-type CBP can interact with CREB to recruit basal transcription machinery and acetylated histone proteins even in the presence of mutant CBP protein. This model is likely to apply to most partial CBP mutations in which wild-type CBP is still present.
Similar articles
- Pharmacological Activators of the NR4A Nuclear Receptors Enhance LTP in a CREB/CBP-Dependent Manner.
Bridi MS, Hawk JD, Chatterjee S, Safe S, Abel T. Bridi MS, et al. Neuropsychopharmacology. 2017 May;42(6):1243-1253. doi: 10.1038/npp.2016.253. Epub 2016 Nov 11. Neuropsychopharmacology. 2017. PMID: 27834392 Free PMC article. - Deacetylase activity is required for cAMP activation of a subset of CREB target genes.
Fass DM, Butler JE, Goodman RH. Fass DM, et al. J Biol Chem. 2003 Oct 31;278(44):43014-9. doi: 10.1074/jbc.M305905200. Epub 2003 Aug 25. J Biol Chem. 2003. PMID: 12939274 - HDAC inhibition modulates hippocampus-dependent long-term memory for object location in a CBP-dependent manner.
Haettig J, Stefanko DP, Multani ML, Figueroa DX, McQuown SC, Wood MA. Haettig J, et al. Learn Mem. 2011 Jan 11;18(2):71-9. doi: 10.1101/lm.1986911. Print 2011 Feb. Learn Mem. 2011. PMID: 21224411 Free PMC article. - Shaping synaptic plasticity: the role of activity-mediated epigenetic regulation on gene transcription.
Cortés-Mendoza J, Díaz de León-Guerrero S, Pedraza-Alva G, Pérez-Martínez L. Cortés-Mendoza J, et al. Int J Dev Neurosci. 2013 Oct;31(6):359-69. doi: 10.1016/j.ijdevneu.2013.04.003. Epub 2013 May 9. Int J Dev Neurosci. 2013. PMID: 23665156 Review. - Histone Deacetylase (HDAC) Inhibitors - emerging roles in neuronal memory, learning, synaptic plasticity and neural regeneration.
Ganai SA, Ramadoss M, Mahadevan V. Ganai SA, et al. Curr Neuropharmacol. 2016;14(1):55-71. doi: 10.2174/1570159x13666151021111609. Curr Neuropharmacol. 2016. PMID: 26487502 Free PMC article. Review.
Cited by
- Synaptic therapy in Alzheimer's disease: a CREB-centric approach.
Teich AF, Nicholls RE, Puzzo D, Fiorito J, Purgatorio R, Fa' M, Arancio O. Teich AF, et al. Neurotherapeutics. 2015 Jan;12(1):29-41. doi: 10.1007/s13311-014-0327-5. Neurotherapeutics. 2015. PMID: 25575647 Free PMC article. Review. - A naturally-occurring histone acetyltransferase inhibitor derived from Garcinia indica impairs newly acquired and reactivated fear memories.
Maddox SA, Watts CS, Doyère V, Schafe GE. Maddox SA, et al. PLoS One. 2013;8(1):e54463. doi: 10.1371/journal.pone.0054463. Epub 2013 Jan 21. PLoS One. 2013. PMID: 23349897 Free PMC article. - Epigenetics and persistent memory: implications for reconsolidation and silent extinction beyond the zero.
Lattal KM, Wood MA. Lattal KM, et al. Nat Neurosci. 2013 Feb;16(2):124-9. doi: 10.1038/nn.3302. Epub 2013 Jan 28. Nat Neurosci. 2013. PMID: 23354385 Free PMC article. - The role of histone acetylation in cocaine-induced neural plasticity and behavior.
Rogge GA, Wood MA. Rogge GA, et al. Neuropsychopharmacology. 2013 Jan;38(1):94-110. doi: 10.1038/npp.2012.154. Epub 2012 Aug 22. Neuropsychopharmacology. 2013. PMID: 22910457 Free PMC article. Review. - Morphine-induced synaptic plasticity in the VTA is reversed by HDAC inhibition.
Authement ME, Langlois LD, Kassis H, Gouty S, Dacher M, Shepard RD, Cox BM, Nugent FS. Authement ME, et al. J Neurophysiol. 2016 Sep 1;116(3):1093-103. doi: 10.1152/jn.00238.2016. Epub 2016 Jun 15. J Neurophysiol. 2016. PMID: 27306674 Free PMC article.
References
- Abel T, Martin KC, Bartsch D, Kandel ER. Memory suppressor genes: inhibitory constraints on the storage of long-term memory. Science. 1998;279:338–341. - PubMed
- Alarcon JM, Malleret G, Touzani K, Vronskaya S, Ishii S, Kandel ER, Barco A. Chromatin acetylation, memory, and LTP are impaired in CBP+/− mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron. 2004;42:947–959. - PubMed
- Barco A, Alarcon JM, Kandel ER. Expression of constitutively active CREB protein facilitates the late phase of long-term potentiation by enhancing synaptic capture. Cell. 2002;108:689–703. - PubMed
- Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 AG000256/AG/NIA NIH HHS/United States
- GM07517/GM/NIGMS NIH HHS/United States
- T32 HL007953/HL/NHLBI NIH HHS/United States
- MH069136/MH/NIMH NIH HHS/United States
- HL07953/HL/NHLBI NIH HHS/United States
- MH060244/MH/NIMH NIH HHS/United States
- T32 GM007517/GM/NIGMS NIH HHS/United States
- R01 MH060244/MH/NIMH NIH HHS/United States
- F31 MH069136/MH/NIMH NIH HHS/United States
- AG00256/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical