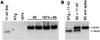Rodent A beta modulates the solubility and distribution of amyloid deposits in transgenic mice - PubMed (original) (raw)
Rodent A beta modulates the solubility and distribution of amyloid deposits in transgenic mice
Joanna L Jankowsky et al. J Biol Chem. 2007.
Abstract
The amino acid sequence of amyloid precursor protein (APP) is highly conserved, and age-related A beta aggregates have been described in a variety of vertebrate animals, with the notable exception of mice and rats. Three amino acid substitutions distinguish mouse and human A beta that might contribute to their differing properties in vivo. To examine the amyloidogenic potential of mouse A beta, we studied several lines of transgenic mice overexpressing wild-type mouse amyloid precursor protein (moAPP) either alone or in conjunction with mutant PS1 (PS1dE9). Neither overexpression of moAPP alone nor co-expression with PS1dE9 caused mice to develop Alzheimer-type amyloid pathology by 24 months of age. We further tested whether mouse A beta could accelerate the deposition of human A beta by crossing the moAPP transgenic mice to a bigenic line expressing human APPswe with PS1dE9. The triple transgenic animals (moAPP x APPswe/PS1dE9) produced 20% more A beta but formed amyloid deposits no faster and to no greater extent than APPswe/PS1dE9 siblings. Instead, the additional mouse A beta increased the detergent solubility of accumulated amyloid and exacerbated amyloid deposition in the vasculature. These findings suggest that, although mouse A beta does not influence the rate of amyloid formation, the incorporation of A beta peptides with differing sequences alters the solubility and localization of the resulting aggregates.
Figures
FIGURE 1. Mouse and human Aβ differ in primary sequence and N-terminal processing
Three amino acid differences at residues 5, 10, and 13 distinguish the rodent and human peptides. These substitutions influence the specificity of BACE1 cleavage. Human APP expressed in transgenic mice is preferentially cleaved at residue 1, producing peptides 38–43 amino acids in length. In contrast, endogenous mouse APP is preferentially cleaved by BACE1 at +11, generating peptides of 28–33 residues. Peptides ending at amino acid 42 are shown for comparison.
FIGURE 2. Transgenic expression increases moAPP 3-fold without lowering co-expressed human APP
Western blots compare mouse and human APP expression in brain homogenates from each of the four genotypes; four animals (2 male and 2 female) from each genotype are shown. Blots show immunodetection of total full-length APP and APP-like protein 2 (top panel, 22C11); rodent-specific APP (second panel, roAPP); human-specific APP (third panel, 6E10); and SOD1 (Cu/Zn superoxide dismutase 1) as an internal control (bottom panel). The 62- and 98-kDa markers in the upper two panels migrated more slowly than expected based on the known size of APP and were consistently positioned lower on the BisTris gels run in MES buffer than in previous studies using Tris-HCl gels in Tris-glycine-SDS buffer.
FIGURE 3. ELISA analysis confirms that transgenic expression of moAPP increases pre-deposit steady-state Aβ levels
A, brain homogenates from each of the lines indicated were assayed at 2.5 (lines 1874, 85, and 1874 × 85) or 6 months of age (line 1874 × S-9) for human-specific Aβ40 (open bars) and Aβ42 (filled bars). As predicted, lines 85 and 1874 × 85 produce identical levels of human peptide. B, measurement of total Aβ (mouse plus human) using the same samples assayed for human Aβ in panel A. Note that Aβ levels in line 1874 × 85 are roughly the sum of Aβ levels in the separate lines. Data are shown ± S.E. *, p < 0.05 versus line 85. †, p < 0.05 versus line 1874.
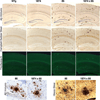
FIGURE 4. Overexpression of mouse APP does not enhance senile plaque pathology in mice producing human Aβ
Amyloid staining by Aβ immunohistochemistry (top row), Campbell-Switzer silver stain (second row), and thioflavine-S (third row) produce qualitatively similar images in 8-month-old mice overexpressing human Aβ (line 85) versus those overexpressing both mouse and human peptide (line 1874 × 85). High power (40×) images of plaques stained by Aβ immunohistochemistry (bottom row, left) and by Hirano silver stain (bottom row, right) suggest that the structure of amyloid aggregates in both lines is also similar. By itself, overexpression of mouse APP (line 1874) failed to produce amyloid plaques at any age examined.
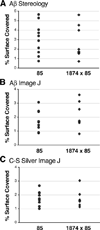
FIGURE 5. Quantitation of senile plaque burden confirms that plaque formation is not increased by the addition of extra mouse Aβ
The panels show scatter plots of the percent surface area covered by amyloid at 8 months of age within the cortex of individual mice for each genotype. Coverage was assessed by two independent methods: non-biased stereology with Stereo-Investigator software (A) or by digital threshold analysis with ImageJ (B and C). Sections used for analysis were stained for amyloid with an anti-Aβ antibody (A and B) or by silver impregnation using the Campbell-Switzer Alzheimer’s stain (C). The three analyses reached similar conclusions: overexpression of mouse Aβ does not change the extent of amyloid formation in the cortex of animals depositing human Aβ.
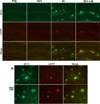
FIGURE 6. Participation of rodent Aβ in amyloid plaques does not increase with overexpression of mouse APP
Double immunostaining for human (top row, 6E10, and green) and mouse Aβ (middle row, roAPP, and red) reveals that human peptide is the dominant species depositing in 8-month-old mice overproducing APP. A minimal core of rodent immunostaining is seen in most plaques; this signal does not increase even when mouse APP/Aβ levels are elevated severalfold (line 85 versus 1874 × 85). The top panels show immunostaining for each genotype at low power; the bottom panels highlight immunostaining in plaques at higher magnification (40×).
FIGURE 7. Overproduction of mouse Aβ increases deposition of human Aβ in cortical blood vessels
A–D, immunostaining for Aβ is more often found within the vasculature of 8-month-old triple transgenic 1874 × 85 mice than in their double transgenic siblings. E, quantitation of amyloid-positive blood vessels in the cortex of Aβ-immunostained sections reveals a significant increase in cerebral amyloid angiopathy in 1874 × 85 mice compared with their line 85 siblings. F–I, amyloid deposits appear in close proximity to, but usually not within cortical blood vessels (indicated by arrowheads) of line 85 animals. J and K, like the parenchymal deposits, vascular amyloid in line 1874 × 85 mice contains both human(E; 6E10) and mouse (F; roAPP) APP/Aβ. Panels A–D and F–I show representative images from three animals for each genotype; all images were taken at 100×. *, p < 0.005 versus line 85.
FIGURE 8. Overexpression of mouse APP increases the solubility of human Aβ aggregates
A, representative example of a filter-trap assay for aggregated Aβ in serial dilutions of brain homogenates from 8-month-old mice. Each row represents a separate mouse. Lacking aggregated peptide, NTg and 1874 homogenates showed no immunostaining for Aβ. Both lines 85 and 1874 × 85 have significant levels of aggregated Aβ, however, less peptide was retained from the 1% SDS homogenates in mice overexpressing mouse and human APP than in those overexpressing only the human protein. B, average intensity of immunostaining within the linear range of the filter-trap dilution series for each genotype reveals a dramatic reduction of aggregated Aβ in the SDS extracts of mice overexpressing mouse and human APP (line 1874 × 85) compared with animals overexpressing only the human protein (line 85). C, Western blot of protein homogenate (lanes 1–6) and extracted filter trap wells (lanes 7–18) probed with human-specific antibody 6E10. Consistent with the greater intensity of staining on the serial dilution filter trap shown in A, the extracted wells from line 85 mice contained more aggregated Aβ than those from 1874 × 85. D, filter-trap quantitation used to generate the genotype averages shown in C are plotted as individual data points. E, separation of genders within each genotype reveals that males carried the lowest, and females the highest, individual amyloid burdens within each group. This is consistent with previous work describing greater amyloid loads in female mice of several other APP transgenic lines (54, 55). *, p < 0.005 versus line 85.
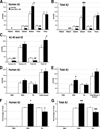
FIGURE 9. ELISA analysis of aggregated Aβ in 8-month-old transgenic mice
Brain homogenates were sequentially extracted with PBS, 2% SDS, and FA before each fraction was assayed for human-specific and total (mouse plus human) Aβ40 and Aβ42. Data from this experiment are tabulated in Table 2. A, accumulation of human Aβ40 was significantly reduced in all three fractions by the expression of exogenous mouse APP. Conversely, human Aβ42 levels increased in the SDS-fraction of triple transgenic mice. B, total Aβ levels (mouse plus human) mirror the differences found in human Aβ. SDS- and FA-soluble Aβ40 was significantly lower, whereas SDS-soluble Aβ42 was significantly higher, in line 1874 × 85 than in line 85. C, overexpression of mouse APP/Aβ significantly decreased the accumulation of both human and total (mouse plus human) Aβ40 summed across all three fractions. The accumulated sum of human Aβ42 is not significantly changed; however, the amount of total (mouse plus human) Aβ42 is substantially higher in the 1874 × 85 mice, suggesting that mouse Aβ42 may account for the extra peptide. D, the two genotypes harbor statistically indistinguishable amounts of human Aβ (40 plus 42) in each fraction and accumulate nearly identical amounts of total human peptide (PBS plus SDS plus FA). E, total mouse plus human Aβ (40 plus 42) differs between the two genotypes only in the SDS-soluble fraction. Despite this increase in SDS-soluble peptide, the overall amount of total Aβ (PBS plus SDS plus FA) in line 1874 × 85 is statistically indistinguishable from line 85. F, although the absolute amount of human Aβ extracted into each fraction is identical in each line, the relative levels differ substantially. A greater fraction of the accumulated Aβ is soluble in SDS in line 1874 × 85 than in line 85. The 1874 × 85 animals show an attendant decrease in the percentage of FA-soluble peptide. G, similar to the case for human Aβ, a greater fraction of total mouse plus human Aβ is soluble in SDS, and correspondingly less in FA, in the 1874 × 85 animals. Data are shown ± S.E. *, p < 0.05; **, p < 0.01; and ***, p < 0.005 versus line 85 by ANOVA/Tukey post-hoc; †, p < 0.05 versus line 85 by Student’s t test (but not by ANOVA).
FIGURE 10. N-terminally truncated mouse Aβ is not a major component of amyloid aggregates
A, immunoprecipitation of Aβ from brain homogenates of 85 and 1874 × 85 mice demonstrates the abundance of full-length Aβ, but fails to detect any sign of N-terminally truncated mouse Aβ 11-x. Peptide was immunoprecipitated and detected with purified 4G8, which binds a mid-region epitope common to both mouse and human Aβ. 5 ng of synthetic human Aβ11–42 was run alongside the IP samples as a positive control. B, immunoprecipitation of synthetic Aβ11–42 spiked into NTg or line 85 brain homogenates provides proof that 4G8 is capable of immunoprecipitating the N-terminally truncated peptide when present. 50 ng of added peptide is shown here, however, as little as 10 ng of exogenous Aβ11–42 could be recovered by immunoprecipitation under conditions identical to those shown in panel A.
Similar articles
- Progressive Spatial Memory Impairment, Brain Amyloid Deposition and Changes in Serum Amyloid Levels as a Function of Age in APPswe/PS1dE9 Mice.
Fu L, Sun Y, Guo Y, Yu B, Zhang H, Wu J, Yu X, Wu H, Kong W. Fu L, et al. Curr Alzheimer Res. 2018;15(11):1053-1061. doi: 10.2174/1567205015666180709112327. Curr Alzheimer Res. 2018. PMID: 29984654 - Receptor-associated protein (RAP) plays a central role in modulating Abeta deposition in APP/PS1 transgenic mice.
Xu G, Karch C, Li N, Lin N, Fromholt D, Gonzales V, Borchelt DR. Xu G, et al. PLoS One. 2008 Sep 8;3(9):e3159. doi: 10.1371/journal.pone.0003159. PLoS One. 2008. PMID: 18776935 Free PMC article. - Episodic-like memory deficits in the APPswe/PS1dE9 mouse model of Alzheimer's disease: relationships to beta-amyloid deposition and neurotransmitter abnormalities.
Savonenko A, Xu GM, Melnikova T, Morton JL, Gonzales V, Wong MP, Price DL, Tang F, Markowska AL, Borchelt DR. Savonenko A, et al. Neurobiol Dis. 2005 Apr;18(3):602-17. doi: 10.1016/j.nbd.2004.10.022. Neurobiol Dis. 2005. PMID: 15755686 - Murine Aβ over-production produces diffuse and compact Alzheimer-type amyloid deposits.
Xu G, Ran Y, Fromholt SE, Fu C, Yachnis AT, Golde TE, Borchelt DR. Xu G, et al. Acta Neuropathol Commun. 2015 Nov 14;3:72. doi: 10.1186/s40478-015-0252-9. Acta Neuropathol Commun. 2015. PMID: 26566997 Free PMC article. - Modeling Alzheimer's disease in transgenic mice: effect of age and of presenilin1 on amyloid biochemistry and pathology in APP/London mice.
Dewachter I, van Dorpe J, Spittaels K, Tesseur I, Van Den Haute C, Moechars D, Van Leuven F. Dewachter I, et al. Exp Gerontol. 2000 Sep;35(6-7):831-41. doi: 10.1016/s0531-5565(00)00149-2. Exp Gerontol. 2000. PMID: 11053674 Review.
Cited by
- Can Animal Models Inform on the Relationship between Depression and Alzheimer Disease?
Nyarko JNK, Quartey MO, Baker GB, Mousseau DD. Nyarko JNK, et al. Can J Psychiatry. 2019 Jan;64(1):18-29. doi: 10.1177/0706743718772514. Epub 2018 Apr 23. Can J Psychiatry. 2019. PMID: 29685068 Free PMC article. Review. - Neuronal Cell Cycle Re-Entry Enhances Neuropathological Features in AppNLF Knock-In Mice.
Barrett T, Stangis KA, Saito T, Saido T, Park KHJ. Barrett T, et al. J Alzheimers Dis. 2021;82(4):1683-1702. doi: 10.3233/JAD-210091. J Alzheimers Dis. 2021. PMID: 34219712 Free PMC article. - Modulation of 5-lipoxygenase in proteotoxicity and Alzheimer's disease.
Valera E, Dargusch R, Maher PA, Schubert D. Valera E, et al. J Neurosci. 2013 Jun 19;33(25):10512-25. doi: 10.1523/JNEUROSCI.5183-12.2013. J Neurosci. 2013. PMID: 23785163 Free PMC article. - Tetrahydrohyperforin prevents cognitive deficit, Aβ deposition, tau phosphorylation and synaptotoxicity in the APPswe/PSEN1ΔE9 model of Alzheimer's disease: a possible effect on APP processing.
Inestrosa NC, Tapia-Rojas C, Griffith TN, Carvajal FJ, Benito MJ, Rivera-Dictter A, Alvarez AR, Serrano FG, Hancke JL, Burgos PV, Parodi J, Varela-Nallar L. Inestrosa NC, et al. Transl Psychiatry. 2011 Jul 12;1(7):e20. doi: 10.1038/tp.2011.19. Transl Psychiatry. 2011. PMID: 22832522 Free PMC article. - Ex vivo analysis platforms for monitoring amyloid precursor protein cleavage.
Kamikubo Y, Jin H, Zhou Y, Niisato K, Hashimoto Y, Takasugi N, Sakurai T. Kamikubo Y, et al. Front Mol Neurosci. 2023 Jan 6;15:1068990. doi: 10.3389/fnmol.2022.1068990. eCollection 2022. Front Mol Neurosci. 2023. PMID: 36683852 Free PMC article.
References
- Selkoe DJ, Bell DS, Podlisny MB, Price DL, Cork LC. Science. 1987;235:873–877. - PubMed
- Struble RG, Price DL, Jr, Cork LC, Price DL. Brain Res. 1985;361:267–275. - PubMed
- Wisniewski HM, Ghetti B, Terry RD. J. Neuropathol. Exp. Neurol. 1973;32:566–584. - PubMed
- Wisniewski H, Johnson AB, Raine CS, Kay WJ, Terry RD. Lab. Invest. 1970;23:287–296. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AG-06-656/AG/NIA NIH HHS/United States
- K01 AG026144-02/AG/NIA NIH HHS/United States
- K01 AG026144-05/AG/NIA NIH HHS/United States
- K01 AG-26144-01/AG/NIA NIH HHS/United States
- K01 AG026144/AG/NIA NIH HHS/United States
- 1 P50 AG-14-248/AG/NIA NIH HHS/United States
- 1 P01 AG-98-003/AG/NIA NIH HHS/United States
- K01 AG026144-04/AG/NIA NIH HHS/United States
- K01 AG026144-01/AG/NIA NIH HHS/United States
- K01 AG026144-03/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous