Adipose tissue-specific inactivation of the retinoblastoma protein protects against diabesity because of increased energy expenditure - PubMed (original) (raw)
Comparative Study
. 2007 Jun 19;104(25):10703-8.
doi: 10.1073/pnas.0611568104. Epub 2007 Jun 7.
Chikage Mataki, Agnès Coste, Nadia Messaddeq, Sylvain Giroud, Stéphane Blanc, Christian Koehl, Marie-France Champy, Pierre Chambon, Lluis Fajas, Daniel Metzger, Kristina Schoonjans, Johan Auwerx
Affiliations
- PMID: 17556545
- PMCID: PMC1965576
- DOI: 10.1073/pnas.0611568104
Comparative Study
Adipose tissue-specific inactivation of the retinoblastoma protein protects against diabesity because of increased energy expenditure
Nassim Dali-Youcef et al. Proc Natl Acad Sci U S A. 2007.
Abstract
The role of the tumor suppressor retinoblastoma protein (pRb) has been firmly established in the control of cell cycle, apoptosis, and differentiation. Recently, it was demonstrated that lack of pRb promotes a switch from white to brown adipocyte differentiation in vitro. We used the Cre-Lox system to specifically inactivate pRb in adult adipose tissue. Under a high-fat diet, pRb-deficient (pRb(ad-/-)) mice failed to gain weight because of increased energy expenditure. This protection against weight gain was caused by the activation of mitochondrial activity in white and brown fat as evidenced by histologic, electron microscopic, and gene expression studies. Moreover, pRb(-/-) mouse embryonic fibroblasts displayed higher proliferation and apoptosis rates than pRb(+/+) mouse embryonic fibroblasts, which could contribute to the altered white adipose tissue morphology. Taken together, our data support a direct role of pRb in adipocyte cell fate determination in vivo and suggest that pRb could serve as a potential therapeutic target to trigger mitochondrial activation in white adipose tissue and brown adipose tissue, favoring an increase in energy expenditure and subsequent weight loss.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
pRb deletion prevents weight gain in mice fed a HFD. (A) HFD induces inguinal and retroperitoneal fat accumulation (∗) in pRbadL2/L2 mice as compared with pRbad−/− animals, in which the white fat furthermore also exhibited a redish aspect. Upon HFD, livers from control mice were pale because of lipid accumulation, whereas pRb-deleted mice livers were dark red. (B) Body weight and dual energy x-ray absorptiometry of body fat content and the percentage of fat 5 days (before HFD) and 6 weeks (after HFD) after tamoxifen to induce adipose-specific pRb deletion (n = 6 animals per group). (C) Body weight progression in pRbadL2/L2 (filled squares) and pRbad−/− mice (open circles) during 45 days of HFD. (D) Comparison of epididymal (epi) WAT, retroperitoneal (retro) WAT, and BAT weights/body weights (BW) between pRbad−/− and pRbadL2/L2 mice. (E) Changes in serum leptin levels between pRbad−/− and control mice after 45 days of HFD. (F) Food intake of pRb-deficient and control mice during 1 month of HFD. (G) Liver weights of pRbad−/− and pRbadL2/L2 mice under HFD. (H) H&E staining of epididymal WAT (a vs. b), retroperitoneal WAT (c vs. d), and BAT sections (g vs. h), immunohistochemistry of WAT sections using an anti-OPA-1 antibody, a mitochondrial protein (e vs. f), and ORO staining of liver sections (i vs. j) in pRbad−/− versus control mice under HFD. ∗, P < 0.05; ∗∗, P < 0.01; §, P < 0.001.
Fig. 2.
pRb deficiency increases energy expenditure. (A) O2 consumption, CO2 production, and total energy expenditure [TEE, calculated from the rate of CO2 production (VCO2) and the respiratory quotient (RQ)] in mutant pRbad−/− (n = 3, open circles) and control pRbadL2/L2 (n = 4, filled squares) mice after 6 weeks on HFD. O2 consumption and CO2 production were normalized to (body weight)0.67 and measured during 12 h in light cycle and 12 h in the dark phase (shaded area). (B) Time course of rectal temperature in pRbad−/− (open circles) and pRbadL2/L2 (filled squares) mice maintained at 4°C. (C) Transmission electron micrographs of osmium tetroxide-stained WAT (a vs. c) and BAT (b vs. d) ultrathin sections of pRbad−/− versus pRbadL2/L2 mice. (Scale bars: a and c, 2 μm; b and d, 1 μm.) ∗ and m, mitochondria; L, lipid droplets. (D) mtDNA content in WAT and BAT of pRbadL2/L2 and pRbad−/− mice (n = 3).
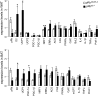
Fig. 3.
Genes expression profiles in WAT and BAT of pRbad−/− mice. Relative mRNA expression levels of the indicated genes in WAT and BAT of pRbad−/− (n = 5) and pRbadL2/L2 (n = 6) mice after HFD. ∗, P < 0.05; ∗∗, P < 0.01. D2, type 2 iodothyronine deiodinase.
Fig. 4.
pRb reduction increases MEFs proliferation and decreases differentiation. (A) Ad-LacZ and Ad-Cre coinfected MEFs stained with β-galactosidase after 12, 24, and 48 h of culture. (B) Relative mRNA expression of pRb in Ad-Cre-infected pRbL2/L2 MEFs at different multiplicities of infection (MOI). (C) FACS analysis of noninfected MEFs and MEFs infected with either Ad-LacZ or Ad-Cre and cultured for 48 h in the presence of a fluorescent CFSE dye. The decrease in CFSE mean fluorescence indicates cell division, and geometric mean fluorescence is inversely proportional to the proliferation rate. CFSE staining diagrams are presented from one representative experiment. (D) ORO staining of terminally differentiated noninfected pRbL2/L2 MEFs and pRbL2/L2 MEFs infected with Ad-LacZ or Ad-Cre.
Fig. 5.
pRb deficiency increases the apoptosis rate. Flow cytometry analysis of apoptosis in (A) noninfected pRbL2/L2 MEFs and Ad-LacZ- and Ad-Cre-infected pRbL2/L2 MEFs and in (B) WAT cell suspension isolated from fat pads of female (F) and male (M) pRbad−/− and pRbadL2/L2 mice 48 h after tamoxifen treatment and staining with annexin V-FITC and PI. The diagrams shown are a representation of three experiments with similar results. ∗, P < 0.05.
Similar articles
- Beige differentiation of adipose depots in mice lacking prolactin receptor protects against high-fat-diet-induced obesity.
Auffret J, Viengchareun S, Carré N, Denis RG, Magnan C, Marie PY, Muscat A, Fève B, Lombès M, Binart N. Auffret J, et al. FASEB J. 2012 Sep;26(9):3728-37. doi: 10.1096/fj.12-204958. Epub 2012 May 25. FASEB J. 2012. PMID: 22637534 - Haploinsufficiency of the retinoblastoma protein gene reduces diet-induced obesity, insulin resistance, and hepatosteatosis in mice.
Mercader J, Ribot J, Murano I, Feddersen S, Cinti S, Madsen L, Kristiansen K, Bonet ML, Palou A. Mercader J, et al. Am J Physiol Endocrinol Metab. 2009 Jul;297(1):E184-93. doi: 10.1152/ajpendo.00163.2009. Epub 2009 May 5. Am J Physiol Endocrinol Metab. 2009. PMID: 19417128 - Retinoblastoma protein functions as a molecular switch determining white versus brown adipocyte differentiation.
Hansen JB, Jørgensen C, Petersen RK, Hallenborg P, De Matteis R, Bøye HA, Petrovic N, Enerbäck S, Nedergaard J, Cinti S, te Riele H, Kristiansen K. Hansen JB, et al. Proc Natl Acad Sci U S A. 2004 Mar 23;101(12):4112-7. doi: 10.1073/pnas.0301964101. Epub 2004 Mar 15. Proc Natl Acad Sci U S A. 2004. PMID: 15024128 Free PMC article. - A new era for brown adipose tissue: New insights into brown adipocyte function and differentiation.
Vila-Bedmar R, Fernández-Veledo S. Vila-Bedmar R, et al. Arch Physiol Biochem. 2011 Jul;117(3):195-208. doi: 10.3109/13813455.2011.560951. Epub 2011 Mar 23. Arch Physiol Biochem. 2011. PMID: 21428723 Review. - The adipose organ: white-brown adipocyte plasticity and metabolic inflammation.
Smorlesi A, Frontini A, Giordano A, Cinti S. Smorlesi A, et al. Obes Rev. 2012 Dec;13 Suppl 2:83-96. doi: 10.1111/j.1467-789X.2012.01039.x. Obes Rev. 2012. PMID: 23107262 Review.
Cited by
- Elevated autophagy gene expression in adipose tissue of obese humans: A potential non-cell-cycle-dependent function of E2F1.
Haim Y, Blüher M, Slutsky N, Goldstein N, Klöting N, Harman-Boehm I, Kirshtein B, Ginsberg D, Gericke M, Guiu Jurado E, Kovsan J, Tarnovscki T, Kachko L, Bashan N, Gepner Y, Shai I, Rudich A. Haim Y, et al. Autophagy. 2015 Nov 2;11(11):2074-2088. doi: 10.1080/15548627.2015.1094597. Autophagy. 2015. PMID: 26391754 Free PMC article. - Control of glutamine metabolism by the tumor suppressor Rb.
Reynolds MR, Lane AN, Robertson B, Kemp S, Liu Y, Hill BG, Dean DC, Clem BF. Reynolds MR, et al. Oncogene. 2014 Jan 30;33(5):556-66. doi: 10.1038/onc.2012.635. Epub 2013 Jan 28. Oncogene. 2014. PMID: 23353822 Free PMC article. - p107 mediated mitochondrial function controls muscle stem cell proliferative fates.
Bhattacharya D, Shah V, Oresajo O, Scimè A. Bhattacharya D, et al. Nat Commun. 2021 Oct 13;12(1):5977. doi: 10.1038/s41467-021-26176-0. Nat Commun. 2021. PMID: 34645816 Free PMC article. - Transcriptional regulatory program in wild-type and retinoblastoma gene-deficient mouse embryonic fibroblasts during adipocyte differentiation.
Hakim-Weber R, Krogsdam AM, Jørgensen C, Fischer M, Prokesch A, Bogner-Strauss JG, Bornstein SR, Hansen JB, Madsen L, Kristiansen K, Trajanoski Z, Hackl H. Hakim-Weber R, et al. BMC Res Notes. 2011 May 26;4:157. doi: 10.1186/1756-0500-4-157. BMC Res Notes. 2011. PMID: 21615920 Free PMC article. - Ablation of LGR4 promotes energy expenditure by driving white-to-brown fat switch.
Wang J, Liu R, Wang F, Hong J, Li X, Chen M, Ke Y, Zhang X, Ma Q, Wang R, Shi J, Cui B, Gu W, Zhang Y, Zhang Z, Wang W, Xia X, Liu M, Ning G. Wang J, et al. Nat Cell Biol. 2013 Dec;15(12):1455-63. doi: 10.1038/ncb2867. Epub 2013 Nov 10. Nat Cell Biol. 2013. PMID: 24212090
References
- Nguyen DX, McCance DJ. J Cell Biochem. 2005;94:870–879. - PubMed
- Lipinski MM, Jacks T. Oncogene. 1999;18:7873–7882. - PubMed
- Chau BN, Wang JY. Nat Rev Cancer. 2003;3:130–138. - PubMed
- Vooijs M, Berns A. Oncogene. 1999;18:5293–5303. - PubMed
- Jacks T, Fazeli A, Schmitt EM, Bronson RT, Goodell MA, Weinberg RA. Nature. 1992;359:295–300. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials