Interferon-gamma induces chronic active myocarditis and cardiomyopathy in transgenic mice - PubMed (original) (raw)
. 2007 Aug;171(2):463-72.
doi: 10.2353/ajpath.2007.060906. Epub 2007 Jun 7.
Hans-Anton Lehr, Michael Torzewski, Gisela Steige, Elena Wiese, Ines Küpper, Christoph Becker, Sibylle Ott, Petra Nusser, Ken-Ichi Yamamura, Gerd Rechtsteiner, Tobias Warger, Andrea Pautz, Hartmut Kleinert, Albrecht Schmidt, Burkert Pieske, Philip Wenzel, Thomas Münzel, Jürgen Löhler
Affiliations
- PMID: 17556594
- PMCID: PMC1934522
- DOI: 10.2353/ajpath.2007.060906
Interferon-gamma induces chronic active myocarditis and cardiomyopathy in transgenic mice
Kurt Reifenberg et al. Am J Pathol. 2007 Aug.
Abstract
Chronic heart failure is associated with an activation of the immune system characterized among other factors by the cardiac synthesis and serum expression of proinflammatory cytokines. There is unequivocal clinical and experimental evidence that the cytokine tumor necrosis factor-alpha is involved in the development of chronic heart failure, but a putative cardiotoxic potential of the proinflammatory cytokine interferon (IFN)-gamma remains primarily unknown. To investigate this issue we analyzed the cardiac phenotype of SAP-IFN-gamma transgenic mice, which constitutively express IFN-gamma in their livers and hence exhibit high circulating serum levels of this cytokine. SAP-IFN-gamma mice spontaneously developed chronic active myocarditis, characterized by the infiltration of not only CD4(+) and CD8(+) T cells but also Mac2(+) (galectin 3(+)) macrophages and CD11c(+) dendritic cells, eventually culminating in cardiomyopathy. Echocardiographic analyses exhibited a left ventricular dilation and impaired systolic function induced by IFN-gamma overexpression. IFN-gamma-mediated cardiotoxicity was associated with high-level cardiac transcription of the proinflammatory cytokines tumor necrosis factor-alpha and interleukin-12 and the macrophage-attracting chemokines MCP1 and MIP1-alpha. Myotoxic IFN-gamma effects could not be detected in smooth or striated muscle tissue, suggesting cardiomyocellular specificity of the toxic IFN-gamma effect. The precise mechanism of IFN-gamma cardiotoxicity remains to be elucidated.
Figures
Figure 1
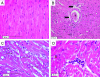
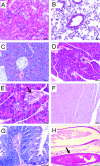
Myocardial lesions in 4-month-old SAP-IFN-γ transgenic mice. The panels show representative myocardial sections (H&E) of SAP-IFN-γ (B–D) and C57BL/6 (B6) mice of an age of 4 months. A: C57BL/6 (B6) mouse with normal myocardial histology. B: The myocardium of SAP-IFN-γ transgenic animals typically exhibits a combination of slight degenerative and mild interstitial inflammatory alterations. Heart muscle fibers vary in their diameters and appear partly shrunken, thereby giving room for a widened (dilated) interstitial space, which becomes especially prominent perivascularly (arrowheads). The asterisk marks the lumen of a penetrating coronary artery with an intact media. Besides degenerating (long black arrows) and regenerating (short black arrows) muscle fibers, paucicellular interstitial infiltrates of mononuclear cells (open arrows) are present. C: At higher magnification, degenerating SAP-IFN-γ muscle fibers appear heavily vacuolated and contain condensed eosinophilic sarcoplasm (arrows). D: A discrete SAP-IFN-γ interstitial infiltrate (asterisk) consisting of mononuclear (lymphoid) cells.
Figure 2
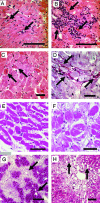
Myocarditis and cardiomyopathy in 6-month-old and moribund SAP-IFN-γ transgenic mice. A–F: Representative longitudinal and cross sections of SAP-IFN-γ transgenic hearts of 6-month-old mice (A–D) and of moribund (E: 10.1-month-old; F: 10.5-month-old) animals. At an age of 6 months, systemic IFN-γ expression had induced in the SAP-IFN-γ hearts (A, B; H&E) a variably intensive cardiac inflammatory reaction characterized by abundant infiltration of mononuclear cells (arrows). The advanced disease process is further characterized by myofiber apoptosis (C, arrows; H&E), cardiomyocellular atrophy and a pronounced fibrosis (D, arrows; periodic acid-Schiff). Cardiomyolyses and interstitial fibrosis were accentuated in the subendocardial ventricular myocardium and in the muscular septum of SAP-IFN-γ hearts. Note the obvious increase of myofiber atrophy and fibrosis in the hearts of moribund animals (E, F; H&E). No cardiac abnormalities could be detected in the age-matched wild-type controls (not shown). G and H: Venous congestion in the livers of moribund 6-month-old SAP-IFN-γ mice (G, H; H&E). Note the characteristic liver alterations such as centrolobular dilation of sinusoids (G, arrows), trabecular atrophy (H, arrows), and centrolobular fatty change (cytoplasmic empty spaces). Scale bars: 200 μm (A, B, E, and G); 100 μm (C, D, F, and H).
Figure 3
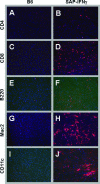
Cellular characterization of SAP-IFN-γ myocarditis. Snap-frozen hearts prepared from 6-month-old wild-type control mice (left) and of SAP-IFN-γ mutants (right) mice were stained with a set of leukocyte-specific antibodies and fluorescent secondary antibodies. Note that the myocarditis of SAP-IFN-γ transgenic mice was characterized by significant infiltration of CD4+ (B) and CD8+ (D) T cells, Mac2+ macrophages (H), and CD11c+ dendritic cells (J), whereas virtually no B220+ B cells (F) contributed to the inflammatory reaction. No specific staining reactions could be found in the hearts of the nontransgenic controls (A, C, E, G, and I). The immunofluorescence analyses were performed with at least two animals per genotype. Pictures presented show typical staining reactions.
Figure 4
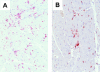
Immunohistochemical demonstration of Mac2+ (=Gal3+) macrophages and CD3+ T cells. Cardiac sections of SAP-IFN-γ mice were immunostained for Mac2 (A) and CD3 (B). Note the significant infiltration of the transgenic myocardium by Mac2+ (=Gal3+) macrophages (A) and CD3+ T cells (B). No such infiltrations could be seen in nontransgenic C57BL/6 controls.
Figure 5
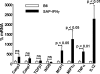
Transcription of proinflammatory cytokines and caspases in SAP-IFN-γ hearts at an age of 6 months. Total RNA was prepared from the hearts of male SAP-IFN-γ (n = 8) and C57BL/6 (n = 8) mice of an age of 6 months and subjected to quantitative RT-PCR reactions as described in Materials and Methods. Relative expression of caspase 3 (Casp3), caspase 7 (casp7), TGF-β1, inducible nitric-oxide synthase (iNOS), MCP1, MIP1-α, TNF-α, and IL-12-p40 mRNA was calculated according to the 2−ΔΔC(T) method. The 2−ΔΔC(T) values of the B6 mice were set to 100%. Note that the SAP-IFN-γ hearts exhibited a significant increase of transcription of the macrophage attracting chemokines MCP1 and MIP1-α and the proinflammatory cytokines TNF-α and IL-12 compared with the C57BL/6 (B6) controls. Casp3 and Casp7 transcription was slightly increased in the SAP-IFN-γ hearts compared with the nontransgenic counterparts; however, these differences did not attain statistical significance. Data of C57BL/6 mice are shown as black bars, SAP-IFN-γ as white bars. The values represent means ± SD (ns, not significant).
Figure 6
Echocardiography. Top: In vivo echocardiographic analysis of cardiac size and function in 6-month-old SAP-IFN-γ transgenic mice (BW, body weight; SEPth, septal wall thickness; LVEDD, left ventricular end diastolic diameter; PWth, posterior wall thickness; LVESD, left ventricular end systolic diameter; FS, percent fractional shortening calculated as [(LVEDD − LVESD)/LVEDD] × 100; LVM, left ventricular mass calculated as [1.04 × (LVEDD + SEPth + PWth)3 −0.8 × LVESD3 + 0.6]; HR, heart rate, all values: mean ± SEM; ns, not significant). Bottom: Representative in vivo M-mode echocardiography of a male 6-month-old SAP-IFN-γ transgenic mouse (right) and a sex- and age-matched C57BL/6 (B6) control (left). Note that the SAP-IFN-γ heart revealed increased LVESD and LVEDD parameters indicating LV dilation and impaired systolic function compared with B6 controls (SEP, interventricular septum; PW, posterior wall).
Figure 7
Effects of transgenic IFN-γ expression on noncardiac organs. Sections (H&E) of various noncardiac organs were prepared from 6-month-old SAP-IFN-γ transgenic (n = 8) and C57BL/6 (n = 8) males and histologically analyzed for pathological lesions. No alterations could be detected in the kidneys (A), lungs (B), femoral musculature (F), duodenum (G), and aorta (H, arrow) of SAP-IFN-γ mice. The pancreas of SAP-IFN-γ mice exhibited foci of a discrete interstitial, mononuclear infiltrate (D: asterisk; E: higher magnification, arrow), whereas no such infiltrates could be observed in the pancreas of the B6 controls (C).
Figure 8
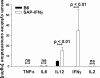
Serum concentration of proinflammatory cytokines. Serum was obtained from 6-month-old male SAP-IFN-γ (n = 9) and C57BL/6 (B6) (n = 9) mice and analyzed for proinflammatory cytokine levels by enzyme-linked immunosorbent assay. Note that the concentrations of IFN-γ and IL-12p40/p70 were significantly enhanced in SAP-IFN-γ mice compared with B6 controls, whereas TNF-α, IL-6, and IL-2 were not. Data of C57BL/6 mice are shown as black bars, SAP-IFN-γ as white bars. The values represent means plus SD (ns, not significant).
Similar articles
- Chronic inflammatory cardiomyopathy of interferon γ-overexpressing transgenic mice is mediated by tumor necrosis factor-α.
Torzewski M, Wenzel P, Kleinert H, Becker C, El-Masri J, Wiese E, Brandt M, Pautz A, Twardowski L, Schmitt E, Münzel T, Reifenberg K. Torzewski M, et al. Am J Pathol. 2012 Jan;180(1):73-81. doi: 10.1016/j.ajpath.2011.09.006. Epub 2011 Nov 1. Am J Pathol. 2012. PMID: 22051774 - Interleukin-12 receptor/STAT4 signaling is required for the development of autoimmune myocarditis in mice by an interferon-gamma-independent pathway.
Afanasyeva M, Wang Y, Kaya Z, Stafford EA, Dohmen KM, Sadighi Akha AA, Rose NR. Afanasyeva M, et al. Circulation. 2001 Dec 18;104(25):3145-51. doi: 10.1161/hc5001.100629. Circulation. 2001. PMID: 11748115 - Cytokine mRNA expression in intestinal tissue of interleukin-2 deficient mice with bowel inflammation.
Autenrieth IB, Bucheler N, Bohn E, Heinze G, Horak I. Autenrieth IB, et al. Gut. 1997 Dec;41(6):793-800. doi: 10.1136/gut.41.6.793. Gut. 1997. PMID: 9462212 Free PMC article. - Inherited Cardiomyopathies Revealed by Clinically Suspected Myocarditis: Highlights From Genetic Testing.
Ader F, Surget E, Charron P, Redheuil A, Zouaghi A, Maltret A, Marijon E, Denjoy I, Hermida A, Fressart V, Gandjbakhch E. Ader F, et al. Circ Genom Precis Med. 2020 Aug;13(4):e002744. doi: 10.1161/CIRCGEN.119.002744. Epub 2020 Jun 10. Circ Genom Precis Med. 2020. PMID: 32522011 Review. No abstract available. - Could interferon-gamma be a therapeutic target for treating heart failure?
Levick SP, Goldspink PH. Levick SP, et al. Heart Fail Rev. 2014 Mar;19(2):227-36. doi: 10.1007/s10741-013-9393-8. Heart Fail Rev. 2014. PMID: 23589353 Free PMC article. Review.
Cited by
- The Abl1 tyrosine kinase is a key player in doxorubicin-induced cardiomyopathy and its p53/p73 cell death mediated signaling differs in atrial and ventricular cardiomyocytes.
Borlak J, Ciribilli Y, Bisio A, Selvaraj S, Inga A, Oh JH, Spanel R. Borlak J, et al. J Transl Med. 2024 Sep 16;22(1):845. doi: 10.1186/s12967-024-05623-8. J Transl Med. 2024. PMID: 39285385 Free PMC article. - Cardiac magnetic resonance and galectin-3 level as predictors of prognostic outcomes for non-ischemic cardiomyopathy patients.
Hu DJ, Xu J, Du W, Zhang JX, Zhong M, Zhou YN. Hu DJ, et al. Int J Cardiovasc Imaging. 2016 Dec;32(12):1725-1733. doi: 10.1007/s10554-016-0958-1. Epub 2016 Aug 26. Int J Cardiovasc Imaging. 2016. PMID: 27566192 - Disease Tolerance and Pathogen Resistance Genes May Underlie Trypanosoma cruzi Persistence and Differential Progression to Chagas Disease Cardiomyopathy.
Chevillard C, Nunes JPS, Frade AF, Almeida RR, Pandey RP, Nascimento MS, Kalil J, Cunha-Neto E. Chevillard C, et al. Front Immunol. 2018 Dec 3;9:2791. doi: 10.3389/fimmu.2018.02791. eCollection 2018. Front Immunol. 2018. PMID: 30559742 Free PMC article. - Biomarkers in Heart Failure: From Research to Clinical Practice.
Berezin AE, Berezin AA. Berezin AE, et al. Ann Lab Med. 2023 May 1;43(3):225-236. doi: 10.3343/alm.2023.43.3.225. Epub 2022 Dec 22. Ann Lab Med. 2023. PMID: 36544334 Free PMC article. Review. - Circulating and Myocardial Cytokines Predict Cardiac Structural and Functional Improvement in Patients With Heart Failure Undergoing Mechanical Circulatory Support.
Diakos NA, Taleb I, Kyriakopoulos CP, Shah KS, Javan H, Richins TJ, Yin MY, Yen CG, Dranow E, Bonios MJ, Alharethi R, Koliopoulou AG, Taleb M, Fang JC, Selzman CH, Stellos K, Drakos SG. Diakos NA, et al. J Am Heart Assoc. 2021 Oct 19;10(20):e020238. doi: 10.1161/JAHA.120.020238. Epub 2021 Oct 1. J Am Heart Assoc. 2021. PMID: 34595931 Free PMC article.
References
- Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling—concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35:569–582. - PubMed
- Devaux B, Scholz D, Hirche A, Klovekorn WP, Schaper J. Upregulation of cell adhesion molecules and the presence of low grade inflammation in human chronic heart failure. Eur Heart J. 1997;18:470–479. - PubMed
- Paulus WJ. Cytokines and heart failure. Heart Fail Monit. 2000;1:50–56. - PubMed
- Mari D, Di Berardino F, Cugno M. Chronic heart failure and the immune system. Clin Rev Allergy Immunol. 2002;23:325–340. - PubMed
- Torre-Amione G. Immune activation in chronic heart failure. Am J Cardiol. 2005;95:3C–40C. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous