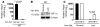Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs) - PubMed (original) (raw)
Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs)
Carole Peyssonnaux et al. J Clin Invest. 2007 Jul.
Abstract
Iron is essential for many biological processes, including oxygen delivery, and its supply is tightly regulated. Hepcidin, a small peptide synthesized in the liver, is a key regulator of iron absorption and homeostasis in mammals. Hepcidin production is increased by iron overload and decreased by anemia and hypoxia; but the molecular mechanisms that govern the hepcidin response to these stimuli are not known. Here we establish that the von Hippel-Lindau/hypoxia-inducible transcription factor (VHL/HIF) pathway is an essential link between iron homeostasis and hepcidin regulation in vivo. Through coordinate downregulation of hepcidin and upregulation of erythropoietin and ferroportin, the VHL-HIF pathway mobilizes iron to support erythrocyte production.
Figures
Figure 1. Iron deficiency downregulates hepcidin in an HIF-1–dependent fashion.
(A) Hepcidin mRNA level in livers of WT mice under regular or low-iron diet (3 weeks), determined by real-time RT-PCR. Results, normalized to 18S ribosomal RNA expression, are expressed as mean ± SD (n = 5 in each group). (B) HIF-1 expression in liver extracts of iron-starved WT mice by Western blotting. (C) Hepcidin mRNA expression in livers of WT and Albumin-Cre/HIF-1_α_flox/flox (HIF-1–/–) iron-starved mice by real-time RT-PCR (n = 8).
Figure 2. Albumin-Cre/VHLflox/flox mice develop erythrocytosis and iron deficiency.
(A) WT and Albumin-Cre/VHLflox/flox (VHL–/–) mice (4 weeks old). Right: Spleen and liver weights of 3- to 4-week-old WT and Albumin-Cre/VHLflox/flox mice (n = 8 in each group). (B) H&E stainings of liver sections from WT and Albumin-Cre/VHLflox/flox mice. Solid arrow indicates steatosis, dashed arrow inflammatory cell infiltrate. (C) EPO mRNA expression in kidney and liver of WT (black bars) and Albumin-Cre/VHLflox/flox (gray bars) mice by real-time RT-PCR (n = 8). EPO, rbc, hematocrit, and hemoglobin levels in blood or serum from 5-week-old mice. n = 8 in each group. (D) Peripheral blood smears from WT and Albumin-Cre/VHLflox/flox mice. Solid arrow indicates hypochromasia, dashed arrow anisocytosis. Right: mean corpuscular hemoglobin (MCH) of WT and Albumin-Cre/VHLflox/flox mice. Original magnification, ×200. (E) Quantification of liver iron level in WT and Albumin-Cre/VHLflox/flox mice using the method of Torrance et al. (33) (n = 5 in each group). (F) Western blot analysis of ferritin in liver extracts from WT and Albumin-Cre/VHLflox/flox mice. (G) Iron staining of splenic sections by Perls Prussian blue. Original magnification, ×200.
Figure 3. Binding of HIF-1 to the promoter of hepcidin and downregulation of hepcidin in Albumin-Cre/VHLflox/flox.
(A) Sequence of murine (C57BL/6) hepcidin promoter; HREs are in bold; arrows indicate primers selected for ChIP. ChIP assay in vivo on liver extracts of WT and Albumin-Cre/VHLflox/flox mice. (B) DFO (150 μM) induces binding of HIF-1 as shown by ChIP assay. (C) Luciferase-reporter constructs under the control of the regulatory region of the human hepcidin gene. HEK293 cells transiently transfected with pGL3 basic or pGL3-Hepc/HRE vector. (D) The “native” (CCACGTG) and mutated (CAA-TG) HREs (indicated by an X) are shown. HEK293 cells were transiently transfected with pGL3 basic, pGL3-Hepc/HRE, or pGL3-Hepc/mutHRE. (E) Hepcidin mRNA expression in livers of WT and Albumin-Cre/VHLflox/flox by real-time RT-PCR (n = 8). HIF-1 and hepcidin expression in liver extracts of WT and Albumin-Cre/VHLflox/flox mice. (F) Hepcidin mRNA expression in livers of WT, Albumin-Cre/VHLflox/flox, and Albumin-Cre/VHLflox/flox/ARNTflox/flox (VHL–/–ANRT–/–) mice (n = 4). (G) IL-6 and IL-1β mRNA levels in livers of WT and Albumin-Cre/VHLflox/flox mice.
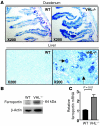
Figure 4. Upregulation of ferroportin in Albumin-Cre/VHLflox/flox mice.
(A) Immunostaining for ferroportin in duodenum and liver sections from WT and Albumin-Cre/VHLflox/flox mice. Solid arrow indicates a hepatocyte, dashed arrow a Kupffer cell. (B) Ferroportin expression in liver extracts of WT and Albumin-Cre/VHLflox/flox mice. (C) Ferroportin mRNA levels in livers of WT and Albumin-Cre/VHLflox/flox mice. Results are expressed as mean ± SD (n ≥ 4 in each group); statistical analysis was done using Student’s t test (unpaired, 2 tailed).
Similar articles
- Hepatic hypoxia-inducible factor-2 down-regulates hepcidin expression in mice through an erythropoietin-mediated increase in erythropoiesis.
Mastrogiannaki M, Matak P, Mathieu JR, Delga S, Mayeux P, Vaulont S, Peyssonnaux C. Mastrogiannaki M, et al. Haematologica. 2012 Jun;97(6):827-34. doi: 10.3324/haematol.2011.056119. Epub 2011 Dec 29. Haematologica. 2012. PMID: 22207682 Free PMC article. - Hypoxia-inducible factor regulates hepcidin via erythropoietin-induced erythropoiesis.
Liu Q, Davidoff O, Niss K, Haase VH. Liu Q, et al. J Clin Invest. 2012 Dec;122(12):4635-44. doi: 10.1172/JCI63924. Epub 2012 Nov 1. J Clin Invest. 2012. PMID: 23114598 Free PMC article. - Evidence for a lack of a direct transcriptional suppression of the iron regulatory peptide hepcidin by hypoxia-inducible factors.
Volke M, Gale DP, Maegdefrau U, Schley G, Klanke B, Bosserhoff AK, Maxwell PH, Eckardt KU, Warnecke C. Volke M, et al. PLoS One. 2009 Nov 18;4(11):e7875. doi: 10.1371/journal.pone.0007875. PLoS One. 2009. PMID: 19924283 Free PMC article. - Regulation of intestinal iron absorption: the mucosa takes control?
Simpson RJ, McKie AT. Simpson RJ, et al. Cell Metab. 2009 Aug;10(2):84-7. doi: 10.1016/j.cmet.2009.06.009. Cell Metab. 2009. PMID: 19656486 Review. - Hepcidin and disorders of iron metabolism.
Ganz T, Nemeth E. Ganz T, et al. Annu Rev Med. 2011;62:347-60. doi: 10.1146/annurev-med-050109-142444. Annu Rev Med. 2011. PMID: 20887198 Review.
Cited by
- Neuronal-specific iron deficiency dysregulates mammalian target of rapamycin signaling during hippocampal development in nonanemic genetic mouse models.
Fretham SJ, Carlson ES, Georgieff MK. Fretham SJ, et al. J Nutr. 2013 Mar;143(3):260-6. doi: 10.3945/jn.112.168617. Epub 2013 Jan 9. J Nutr. 2013. PMID: 23303869 Free PMC article. - HIF-α Prolyl Hydroxylase Inhibitors and Their Implications for Biomedicine: A Comprehensive Review.
Hirota K. Hirota K. Biomedicines. 2021 Apr 24;9(5):468. doi: 10.3390/biomedicines9050468. Biomedicines. 2021. PMID: 33923349 Free PMC article. Review. - Alternatives to blood transfusion.
Spahn DR, Goodnough LT. Spahn DR, et al. Lancet. 2013 May 25;381(9880):1855-65. doi: 10.1016/S0140-6736(13)60808-9. Lancet. 2013. PMID: 23706802 Free PMC article. Review. - Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2.
Prabhakar NR, Semenza GL. Prabhakar NR, et al. Physiol Rev. 2012 Jul;92(3):967-1003. doi: 10.1152/physrev.00030.2011. Physiol Rev. 2012. PMID: 22811423 Free PMC article. Review. - Iron in the Tumor Microenvironment-Connecting the Dots.
Pfeifhofer-Obermair C, Tymoszuk P, Petzer V, Weiss G, Nairz M. Pfeifhofer-Obermair C, et al. Front Oncol. 2018 Nov 26;8:549. doi: 10.3389/fonc.2018.00549. eCollection 2018. Front Oncol. 2018. PMID: 30534534 Free PMC article. Review.
References
- Nemeth E., et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases