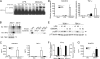The chemotherapeutic agent DMXAA potently and specifically activates the TBK1-IRF-3 signaling axis - PubMed (original) (raw)
. 2007 Jul 9;204(7):1559-69.
doi: 10.1084/jem.20061845. Epub 2007 Jun 11.
Nadege Goutagny, Pin-Yu Perera, Hiroki Kato, Himanshu Kumar, Taro Kawai, Shizuo Akira, Ram Savan, David van Echo, Katherine A Fitzgerald, Howard A Young, Lai-Ming Ching, Stefanie N Vogel
Affiliations
- PMID: 17562815
- PMCID: PMC2118649
- DOI: 10.1084/jem.20061845
The chemotherapeutic agent DMXAA potently and specifically activates the TBK1-IRF-3 signaling axis
Zachary J Roberts et al. J Exp Med. 2007.
Abstract
Vascular disrupting agents (VDAs) represent a novel approach to the treatment of cancer, resulting in the collapse of tumor vasculature and tumor death. 5,6-dimethylxanthenone-4-acetic acid (DMXAA) is a VDA currently in advanced phase II clinical trials, yet its precise mechanism of action is unknown despite extensive preclinical and clinical investigations. Our data demonstrate that DMXAA is a novel and specific activator of the TANK-binding kinase 1 (TBK1)-interferon (IFN) regulatory factor 3 (IRF-3) signaling pathway. DMXAA treatment of primary mouse macrophages resulted in robust IRF-3 activation and approximately 750-fold increase in IFN-beta mRNA, and in contrast to the potent Toll-like receptor 4 (TLR4) agonist lipopolysaccharide (LPS), signaling was independent of mitogen-activated protein kinase (MAPK) activation and elicited minimal nuclear factor kappaB-dependent gene expression. DMXAA-induced signaling was critically dependent on the IRF-3 kinase, TBK1, and IRF-3 but was myeloid differentiation factor 88-, Toll-interleukin 1 receptor domain-containing adaptor inducing IFN-beta-, IFN promoter-stimulator 1-, and inhibitor of kappaB kinase-independent, thus excluding all known TLRs and cytosolic helicase receptors. DMXAA pretreatment of mouse macrophages induced a state of tolerance to LPS and vice versa. In contrast to LPS stimulation, DMXAA-induced IRF-3 dimerization and IFN-beta expression were inhibited by salicylic acid. These findings detail a novel pathway for TBK1-mediated IRF-3 activation and provide new insights into the mechanism of this new class of chemotherapeutic drugs.
Figures
Figure 1.
DMXAA preferentially induces IRF-3–mediated gene expression. (A) Peritoneal macrophages from C57BL/6 mice were stimulated for 2 h with 100 ng/ml LPS or increasing concentrations of DMXAA, as shown. Total RNA was extracted and subjected to reverse transcription followed by quantitative real-time PCR, as described in Materials and methods. (B) Primary mouse macrophages were stimulated with medium alone, 100 ng/ml LPS, or 100 μg/ml DMXAA for the indicated times. Total protein was collected and subjected to SDS-PAGE, followed by Western blotting with anti-IκBα antibody. Protein molecular masses (in kD) appear at the right. (C) RAW 264.7 macrophages were stimulated with medium alone, 10 ng/ml LPS, or 1 mg/ml DMXAA for the indicated times. Nuclear extracts were prepared and subjected to EMSA with an NF-κB–specific labeled oligonucleotide. (D) Peritoneal macrophages from C57BL/6 mice were stimulated with medium alone, 100 ng/ml LPS, or 100 μg/ml DMXAA for the indicated times. Western blotting for activated MAPK was performed on whole-cell lysates. β-Actin was used as a loading control. Protein masses (in kD) appear at the right. (E) Peritoneal macrophages from TLR4+/+ and TLR4−/− were stimulated with medium alone, 100 ng/ml LPS, or 100 μg/ml DMXAA for 2 h. Total RNA was collected and analyzed by quantitative real-time PCR, as in A. Results represent the mean ± SE for at least three independent experiments. **, P < 0.01.
Figure 2.
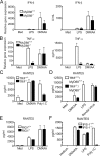
DMXAA is a potent and specific activator of TBK1. (A) Peritoneal macrophages from C57BL/6 mice were stimulated with medium alone, 100 ng/ml LPS, or 100 μg/ml DMXAA for the indicated times. Total protein was collected and subjected to native PAGE, followed by Western blotting with an anti–IRF-3 antibody. (B) Bone marrow–derived macrophages were stimulated for 90 min with medium alone, 200 ng/ml LPS, or 100 μg/ml DMXAA. Whole-cell lysates were subjected to immunoprecipitation with anti-TBK1 pAb, and immunoprecipitates were subjected to an in vitro kinase assay using the GST–C-terminal IRF-3 wild-type or A7 mutant as the substrate, as described in Materials and methods. Levels of TBK1 in immunoprecipitates were examined by Western blotting using anti-TBK1 mAb. Recombinant TBK1 and IKKβ were also included as controls with GST–IRF-3 or IκBα substrates. Protein molecular mass appears at the right. Results shown are representative of four independent experiments. (C) Peritoneal macrophages from IRF-3+/+ and IRF-3−/− were exposed to medium alone or 100 μg/ml DMXAA for 24 h. Supernatants were collected, and cytokine concentrations were determined by Luminex bead assay. Results are representative of three independent experiments. (D) TBK1+/+ and TBK1−/− MEFs were stimulated with medium alone, 100 ng/ml LPS, or 100 μg/ml DMXAA for 2 h. Total RNA was harvested and subjected to reverse transcription. Relative gene expression was measured by quantitative real-time PCR. Results represent the mean ± SE of at least three separate experiments. (E) TBK1+/+ and TBK1−/− MEFs were stimulated with 10 μg/ml LPS or 100 μg/ml DMXAA for 10 or 60 min. Whole-cell lysates were subjected to SDS-PAGE and probed with the indicated antibodies. Protein molecular masses appear at the right. Results are representative of three independent experiments. (F) IKKβ+/+ and IKKβ−/− MEFs were stimulated for 3 h with medium alone, 100 μg/ml DMXAA, 10 μg/ml or poly I:C in the presence of Fugene 6. IFN-β expression was measured by real-time PCR. Results are representative of three independent experiments. (G) Peritoneal macrophages isolated from IKKɛ+/+ or IKKɛ−/− mice were stimulated with 100 μg/ml DMXAA for 24 h. Supernatants were collected, and RANTES expression was determined by ELISA. Results shown are mean ± SD for three independent experiments.
Figure 3.
DMXAA signaling is TLR- and IPS-1–independent. (A and B) Peritoneal macrophages from MyD88+/+ or MyD88−/− mice were stimulated with 100 ng/ml LPS or 100 μg/ml DMXAA. After a 2-h incubation (left), total RNA was collected, and gene expression was assessed by quantitative real-time PCR. ELISA results (right) were generated from supernatants collected 24 h after exposure to medium, 100 ng/ml LPS, or 100 μg/ml DMXAA. (C) TRIF+/+ and TRIF−/− MEFs were stimulated with 100 μg/ml DMXAA or 100 μg/ml poly I:C for 24 h. Supernatants were collected, and RANTES levels were measured by ELISA. (D) Wild-type or MyD88−/−/TRIF−/− peritoneal macrophages were stimulated with 100 mg/ml DMXAA, 100 ng/ml LPS, or 200 hemagglutination U/ml Sendai virus for 24 h. RANTES expression was measured by ELISA. RIG-I+/+ and RIG-I−/− (E) or IPS-1+/+ and IPS-1−/− (F) MEFs were treated for 24 h with medium alone, 1 μg/ml LPS, 100 μg/ml DMXAA, or 10 μg/ml poly I:C in the presence of Fugene 6. Supernatants were collected, and RANTES levels were assessed by ELISA. Results shown are the mean ± SE of at least three independent experiments. **, P < 0.01; ***, P < 0.001.
Figure 4.
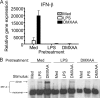
DMXAA pretreatment of macrophages induces a state of refractoriness upon reexposure to either LPS or DMXAA. Total RNA (A) or protein (B) from C57BL/6 peritoneal macrophages pretreated with medium alone, 100 ng/ml LPS, or 100 μg/ml DMXAA, washed, and restimulated with medium, LPS, or DMXAA was collected and subjected to quantitative real-time PCR (A) or native PAGE, followed by Western blotting for IRF-3 (B). Results shown are the mean ± SE of one representative experiment (n = 3).
Figure 5.
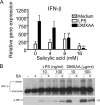
Sensitivity of DMXAA-induced gene expression and IRF-3 activation to SA. (A) Peritoneal macrophages from C57BL/6 mice were pretreated with medium alone or increasing concentrations of SA for 1 h and stimulated with LPS or DMXAA for an additional 2 h. Total RNA was harvested, and gene expression was assessed by quantitative PCR. Results represent the mean ± SE of at least three separate experiments. **, P < 0.01; ***, P < 0.001. (B) Macrophages were pretreated with or without 16 mM SA for 1 h and stimulated with 10 or 100 ng/ml LPS, or 10 or 100 μg/ml DMXAA, for an additional 1 h. Total protein was harvested and subjected to native PAGE (top) or SDS-PAGE (bottom), followed by Western blot analysis for IRF-3 and anti–phospho–IRF-3, respectively. Protein molecular mass appears at the right. Results shown are representative of three independent experiments.
Similar articles
- The TRIF/TBK1/IRF-3 activation pathway is the primary inhibitory target of resveratrol, contributing to its broad-spectrum anti-inflammatory effects.
Kim MH, Yoo DS, Lee SY, Byeon SE, Lee YG, Min T, Rho HS, Rhee MH, Lee J, Cho JY. Kim MH, et al. Pharmazie. 2011 Apr;66(4):293-300. Pharmazie. 2011. PMID: 21612158 - Involvement of TBK1 and IKKepsilon in lipopolysaccharide-induced activation of the interferon response in primary human macrophages.
Solis M, Romieu-Mourez R, Goubau D, Grandvaux N, Mesplede T, Julkunen I, Nardin A, Salcedo M, Hiscott J. Solis M, et al. Eur J Immunol. 2007 Feb;37(2):528-39. doi: 10.1002/eji.200636090. Eur J Immunol. 2007. PMID: 17236232 - Mechanism of inhibition of lipopolysaccharide-induced interferon-β production by 2-aminopurine.
Sugiyama T, Gotou T, Moriyama K, Kajiura N, Hasegawa T, Tomida J, Takahashi K, Komatsu T, Ueda H, Sato K, Tokoro S, Neri P, Mori H. Sugiyama T, et al. Mol Immunol. 2012 Oct;52(3-4):299-304. doi: 10.1016/j.molimm.2012.06.008. Epub 2012 Jul 1. Mol Immunol. 2012. PMID: 22750230 - Interferon response induced by Toll-like receptor signaling.
Takeuchi O, Hemmi H, Akira S. Takeuchi O, et al. J Endotoxin Res. 2004;10(4):252-6. doi: 10.1179/096805104225005896. J Endotoxin Res. 2004. PMID: 15373970 Review. - Mechanisms and pathways of innate immune activation and regulation in health and cancer.
Cui J, Chen Y, Wang HY, Wang RF. Cui J, et al. Hum Vaccin Immunother. 2014;10(11):3270-85. doi: 10.4161/21645515.2014.979640. Hum Vaccin Immunother. 2014. PMID: 25625930 Free PMC article. Review.
Cited by
- 5,6-Dimethylxanthenone-4-acetic acid (DMXAA) activates stimulator of interferon gene (STING)-dependent innate immune pathways and is regulated by mitochondrial membrane potential.
Prantner D, Perkins DJ, Lai W, Williams MS, Sharma S, Fitzgerald KA, Vogel SN. Prantner D, et al. J Biol Chem. 2012 Nov 16;287(47):39776-88. doi: 10.1074/jbc.M112.382986. Epub 2012 Oct 1. J Biol Chem. 2012. PMID: 23027866 Free PMC article. - cGAS-STING pathway in cancer biotherapy.
Wang Y, Luo J, Alu A, Han X, Wei Y, Wei X. Wang Y, et al. Mol Cancer. 2020 Sep 4;19(1):136. doi: 10.1186/s12943-020-01247-w. Mol Cancer. 2020. PMID: 32887628 Free PMC article. Review. - Refractoriness of interferon-beta signaling through NOD1 pathway in mouse respiratory epithelial cells using the anticancer xanthone compound.
Yu Z, Predina JD, Cheng G. Yu Z, et al. World J Biol Chem. 2013 May 26;4(2):18-29. doi: 10.4331/wjbc.v4.i2.18. World J Biol Chem. 2013. PMID: 23710296 Free PMC article. - Labeling of oxidizable proteins with a photoactivatable analog of the antitumor agent DMXAA: evidence for redox signaling in its mode of action.
Brauer R, Wang LC, Woon ST, Bridewell DJ, Henare K, Malinger D, Palmer BD, Vogel SN, Kieda C, Tijono SM, Ching LM. Brauer R, et al. Neoplasia. 2010 Sep;12(9):755-65. doi: 10.1593/neo.10636. Neoplasia. 2010. PMID: 20824052 Free PMC article. - NOD2, RIP2 and IRF5 play a critical role in the type I interferon response to Mycobacterium tuberculosis.
Pandey AK, Yang Y, Jiang Z, Fortune SM, Coulombe F, Behr MA, Fitzgerald KA, Sassetti CM, Kelliher MA. Pandey AK, et al. PLoS Pathog. 2009 Jul;5(7):e1000500. doi: 10.1371/journal.ppat.1000500. Epub 2009 Jul 3. PLoS Pathog. 2009. PMID: 19578435 Free PMC article.
References
- Shankaran, V., H. Ikeda, A.T. Bruce, J.M. White, P.E. Swanson, L.J. Old, and R.D. Schreiber. 2001. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 410:1107–1111. - PubMed
- Pace, J.L., S.W. Russell, P.A. LeBlanc, and D.M. Murasko. 1985. Comparative effects of various classes of mouse interferons on macrophage activation for tumor cell killing. J. Immunol. 134:977–981. - PubMed
- Tsung, K., J.P. Dolan, Y.L. Tsung, and J.A. Norton. 2002. Macrophages as effector cells in interleukin 12-induced T cell-dependent tumor rejection. Cancer Res. 62:5069–5075. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 AI 007540/AI/NIAID NIH HHS/United States
- R01 AI044936/AI/NIAID NIH HHS/United States
- R37 AI067497/AI/NIAID NIH HHS/United States
- R01 AI067497/AI/NIAID NIH HHS/United States
- R56 AI067497/AI/NIAID NIH HHS/United States
- T32 AI007540/AI/NIAID NIH HHS/United States
- R37 AI018797/AI/NIAID NIH HHS/United States
- AI 18797/AI/NIAID NIH HHS/United States
- AI 067497/AI/NIAID NIH HHS/United States
- R01 AI018797/AI/NIAID NIH HHS/United States
- AI 44936/AI/NIAID NIH HHS/United States
- R56 AI018797/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous