Functional consequences of alteration of N-linked glycosylation sites on the neurokinin 1 receptor - PubMed (original) (raw)
Comparative Study
. 2007 Jun 19;104(25):10691-6.
doi: 10.1073/pnas.0703394104. Epub 2007 Jun 11.
Affiliations
- PMID: 17563389
- PMCID: PMC1965574
- DOI: 10.1073/pnas.0703394104
Comparative Study
Functional consequences of alteration of N-linked glycosylation sites on the neurokinin 1 receptor
Morris F Tansky et al. Proc Natl Acad Sci U S A. 2007.
Abstract
The neurokinin 1 receptor (NK1R), a G protein-coupled receptor involved in diverse functions including pain and inflammation, has two putative N-linked glycosylation sites, Asn-14 and Asn-18. We studied the role of N-linked glycosylation in the functioning of the NK1R by constructing three receptor mutants: two single mutants (Asn --> Gln-14 and Asn --> Gln-18) and a double mutant, lacking both glycosylation sites. Using a lentiviral transfection system, the mutants were stably transfected into NCM 460 cells, a nontransformed human colonic epithelial cell line. We observed that the magnitude of glycosylation as estimated by changes in gel migration depends on the number of glycosylation sites available, with the wild-type receptor containing the greatest amount of glycosylation. All mutant receptors were able to bind to substance P and neurokinin A ligand with similar affinities; however, the double mutant, nonglycosylated NK1R showed only half the B(max) of the wild-type NK1R. In terms of receptor function, the ablation of both N-linked glycosylation sites did not have a profound effect on the receptors' abilities to activate the MAP kinase families (p42/p44, JNK, and p38), but did affect SP-induced IL-8 secretion. All mutants were able to internalize, but the kinetics of internalization of the double mutant receptor was more rapid, when compared with wild-type NK1R. Therefore, glycosylation of NK1R may stabilize the receptor in the plasma membrane. These results contribute to the ongoing elucidation of the role of glycosylation in G protein-coupled receptors and the study of the neurokinin receptors in particular.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
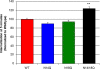
Extent of glycosylation and expression of wild-type and mutant receptors. (A) NK1R immunoblot of transfected NCM 460 cell lysates shows the nonglycosylated mutant receptors migrate at a lower relative mass than the wild-type NK1R, reflecting a loss of carbohydrate moieties. (B) Real-time PCR comparison of lentiviral mutants in NCM 460 cells, demonstrating equal NK1R message for the NCM 460 cells transfected with the various glycosylation mutants (α = 0.05, P > 0.05). NCM 460 NK1R mRNA levels were normalized to TATA-box binding protein mRNA. The values are expressed as a percentage of the wild-type NK1R mRNA abundance.
Fig. 2.
Competition binding and 125I-BH-SP saturation analyses of wild-type and glycosylation receptor mutants in NCM 460 cells. These analyses demonstrate that there are no effects of glycosylation on the _K_i of SP in the presence of 125I-BH-SP (A; competition binding analysis), but that there is a 48% decrease in the _B_max of the nonglycosylated mutant when compared with the other mutants and wild-type (B; saturation analysis).
Fig. 3.
NK1R immunostaining of NCM 460 cells transfected with wild-type and mutant NK1 receptors, after stimulation by SP for the indicated durations. Untreated cells containing wild-type or mutant receptors show NK1R immunoreactivity on the cell surface, whereas SP stimulation triggers NK1R internalization, visualized as punctate staining (closed arrows). After longer SP stimulation, the mutant receptors are still retained within the cell, in contrast to the wild-type receptors that have returned to the cell surface (open arrows) by 45 min. Representative images of each cell line are shown. (Scale bar: 20 μm.)
Fig. 4.
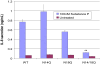
Internalization kinetics of NK-1R mutants. Cells preincubated with 125I-BH-SP for 1 h at 4°C were warmed to 37°C for the indicated times. Cells were acid-washed to remove surface-bound ligand and treated with alkaline wash to determine the amount of internalized ligand by a gamma counter. The double glycosylation receptor mutant displays a significant 24% increase in the relative amount of internalized receptor when compared with the wild-type receptor (α = 0.01, P < 0.01).
Fig. 5.
Comparison of SP-induced phosphorylation of MAPK pathways p38, p42/p44, and JNK in cell lines transfected with NK1R mutants. Immunoblot shows the duration of SP-mediated activation of p38, p42/p44, and JNK in all cell lines. p38 and p42/p44 are phosphorylated within 5 min of SP incubation, whereas JNK phosphorylation occurs within 30 min. Wild-type and mutant receptors exhibit similar phosphorylation patterns. Total p42/p44 (shown), total p38, and total JNK were also assayed to assure loading equivalency. Representative immunoblots from independent experiments are shown.
Fig. 6.
Immunoblots demonstrating SP-specific effect of MAPK phosphorylation, using NK1R antagonist CJ12,255. Pretreatment of cells with the NK1R antagonist ablates p42/p44 and JNK phosphorylation induced by SP (purple: SP alone; orange: antagonist plus SP) at the indicated times, suggesting that SP acts solely through NK1R to activate the MAPK family.
Fig. 7.
Transfected NCM 460 cells were stimulated for 4 h with 100 nM SP, demonstrating by ELISA a pronounced diminution of IL-8 production in the N14Q/N18Q mutant cell line lacking both glycosylation sites. This reduction was significant when compared with cells containing the wild-type and single mutant receptors (α = 0.01, P < 0.001).
Similar articles
- Substance P Receptor Antagonist Suppresses Inflammatory Cytokine Expression in Human Disc Cells.
Kepler CK, Markova DZ, Koerner JD, Mendelis J, Chen CM, Vaccaro AR, Risbud MV, Albert TJ, Anderson DG. Kepler CK, et al. Spine (Phila Pa 1976). 2015 Aug 15;40(16):1261-9. doi: 10.1097/BRS.0000000000000954. Spine (Phila Pa 1976). 2015. PMID: 25929203 - Roles of full-length and truncated neurokinin-1 receptors on tumor progression and distant metastasis in human breast cancer.
Zhou Y, Zhao L, Xiong T, Chen X, Zhang Y, Yu M, Yang J, Yao Z. Zhou Y, et al. Breast Cancer Res Treat. 2013 Jul;140(1):49-61. doi: 10.1007/s10549-013-2599-6. Epub 2013 Jun 27. Breast Cancer Res Treat. 2013. PMID: 23807418 - Differential consequences of neurokinin receptor 1 and 2 antagonists in metastatic breast carcinoma cells; Effects independent of Substance P.
Nizam E, Erin N. Nizam E, et al. Biomed Pharmacother. 2018 Dec;108:263-270. doi: 10.1016/j.biopha.2018.09.013. Epub 2018 Sep 14. Biomed Pharmacother. 2018. PMID: 30223097 - Substance P and the Neurokinin-1 Receptor: The New CRF.
Schank JR, Heilig M. Schank JR, et al. Int Rev Neurobiol. 2017;136:151-175. doi: 10.1016/bs.irn.2017.06.008. Epub 2017 Aug 18. Int Rev Neurobiol. 2017. PMID: 29056150 Review. - New insight into the role of substance P/neurokinin-1 receptor system in breast cancer progression and its crosstalk with microRNAs.
Ebrahimi S, Javid H, Alaei A, Hashemy SI. Ebrahimi S, et al. Clin Genet. 2020 Oct;98(4):322-330. doi: 10.1111/cge.13750. Epub 2020 Apr 20. Clin Genet. 2020. PMID: 32266968 Review.
Cited by
- The single kinin receptor signals to separate and independent physiological pathways in Malpighian tubules of the yellow fever mosquito.
Schepel SA, Fox AJ, Miyauchi JT, Sou T, Yang JD, Lau K, Blum AW, Nicholson LK, Tiburcy F, Nachman RJ, Piermarini PM, Beyenbach KW. Schepel SA, et al. Am J Physiol Regul Integr Comp Physiol. 2010 Aug;299(2):R612-22. doi: 10.1152/ajpregu.00068.2010. Epub 2010 Jun 10. Am J Physiol Regul Integr Comp Physiol. 2010. PMID: 20538895 Free PMC article. - The N-terminal region of the dopamine D2 receptor, a rhodopsin-like GPCR, regulates correct integration into the plasma membrane and endocytic routes.
Cho DI, Min C, Jung KS, Cheong SY, Zheng M, Cheong SJ, Oak MH, Cheong JH, Lee BK, Kim KM. Cho DI, et al. Br J Pharmacol. 2012 May;166(2):659-75. doi: 10.1111/j.1476-5381.2011.01787.x. Br J Pharmacol. 2012. PMID: 22117524 Free PMC article. - MicroRNA-31-3p Is Involved in Substance P (SP)-Associated Inflammation in Human Colonic Epithelial Cells and Experimental Colitis.
Fang K, Law IKM, Padua D, Sideri A, Huang V, Kevil CG, Iliopoulos D, Pothoulakis C. Fang K, et al. Am J Pathol. 2018 Mar;188(3):586-599. doi: 10.1016/j.ajpath.2017.10.023. Epub 2017 Dec 16. Am J Pathol. 2018. PMID: 29253460 Free PMC article. - Denatured G-protein coupled receptors as immunogens to generate highly specific antibodies.
Talmont F, Moulédous L, Boué J, Mollereau C, Dietrich G. Talmont F, et al. PLoS One. 2012;7(9):e46348. doi: 10.1371/journal.pone.0046348. Epub 2012 Sep 27. PLoS One. 2012. PMID: 23029489 Free PMC article. - Antibodies against G-protein coupled receptors: novel uses in screening and drug development.
Gupta A, Heimann AS, Gomes I, Devi LA. Gupta A, et al. Comb Chem High Throughput Screen. 2008 Jul;11(6):463-7. doi: 10.2174/138620708784911465. Comb Chem High Throughput Screen. 2008. PMID: 18673273 Free PMC article. Review.
References
- Pothoulakis C, Castagliuolo I, Leeman SE, Wang CC, Li H, Hoffman BJ, Mezey E. Am J Physiol. 1998;275:G68–G75. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials