Retrograde axonal transport: a major transmission route of enterovirus 71 in mice - PubMed (original) (raw)
Retrograde axonal transport: a major transmission route of enterovirus 71 in mice
Che-Szu Chen et al. J Virol. 2007 Sep.
Abstract
Inoculation of enterovirus 71 (EV71) by the oral (p.o.), intramuscular (i.m.), or intracranial route resulted in brain infection, flaccid paralysis, pulmonary dysfunction, and death of 7-day-old mice. The lag time of disease progression indicated that neuroinvasion from the inoculation sites was a prerequisite for the development of the clinical signs. Although EV71 p.o. inoculation led to a persistent viremia and a transient increase in blood-brain barrier permeability at the early stage of the infection, only low levels of virus, which led to neither severe infection nor clinical illness, could be detected in the brain, suggesting that hematogenous transport might not represent a major transmission route. In the spinal cord, following both p.o. and hind limb i.m. inoculation, the virus first appeared and increased rapidly in the lower segments, especially at the anterior horn areas, and then spread to the upper segments and brain in the presence of viremia. A reverse pattern, with the virus being first detected in the upper segment, was observed when the virus was i.m. inoculated in the forelimb. Colchicine, a fast axonal transport inhibitor, but not sciatic nerve transection reduced EV71 neuroinvasion in a dose-dependent manner, indicating a neuronal transmission of the virus.
Figures
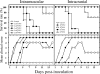
FIG. 1.
i.m. and i.c. inoculation of EV71 result in dose-related paralysis and mortality. Seven-day-old ICR mice (n = 12) were i.m. (2 × 101 to 2 × 105 PFU/mouse; hind limb) or i.c. (102 to 106 PFU/mouse) inoculated with strain EV71/MP4. The survival rate was then monitored daily after infection. Clinical disease was scored as follows: 0, healthy; 1, ruffled hair, hunchbacked, or reduced mobility; 2, wasting; 3, limb weakness; 4, limb paralysis; and 5, death.
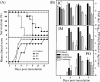
FIG. 2.
The inoculation route determines the disease progression of EV71-infected mice. ICR mice (n = 12) were i.c., i.m. (hind limb), or p.o. inoculated with strain EV71/MP4 at 5 × 104, 5 × 103, and 5 × 106 PFU/mouse, respectively. Survival rate and clinical disease (A), and tissue viral load (B) were determined after infection. Results are the means and SEM.
FIG. 3.
The development of pulmonary dysfunction in EV71-infected mice is related to the inoculation routes. ICR mice (n = 12) were inoculated with EV71/MP4 i.c., i.m., or p.o. as described in the legend to Fig. 2. Peak inspiratory flow (PIF) and tidal volume (TV) were then monitored by whole-body plethysmography. Results are the means and SEM.
FIG. 4.
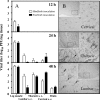
i.c. EV71-inoculated mice develop brain lesions and emphysema at the late stage of infection. ICR mice were i.c. inoculated with EV71/MP4 (5 × 104 PFU/mouse). Brain and lung tissues were collected for general morphology (hematoyxlin and eosin stain) and immunohistochemical staining with anti-EV71 MAb. EV71 antigen (A and B, arrows) and neuropil vacuolation and myelin balls (C, arrowheads) occur in areas under the cerebellar peduncles. Magnifications, ×40 (insets in panels A, B, and C), ×200 (D, E, and F), and ×400 (A, B, and C).
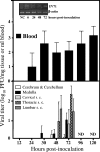
FIG. 5.
p.o. EV71 inoculation results in viremia without severe neuroinvasion early after infection. ICR mice (n = 12 to 18) were p.o. inoculated with EV71/MP4 (5 × 106 PFU/mouse). The viral titers in the blood and CNS were determined by plaque assay. Results are the means and SEM. s. c., spinal cord; ND, not determined. Inset, detection of the EV71 genome in leukocytes. Agarose gel electrophoresis of the ethidium bromide-stained products of reverse transcriptase PCRs using RNA extracted from leukocytes of EV71-infected mice is shown. NC, sample without RNA.
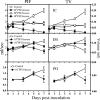
FIG. 6.
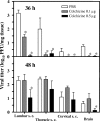
EV71 neuroinvasion after i.m. inoculation. ICR mice (n = 6 to 12) were i.m. inoculated with EV71/MP4 (5 × 103 PFU/mouse) into either the left hind limb or forelimb. (A) The tissue viral titer was determined by plaque assay. Results are the means and SEM. s. c., spinal cord. (B) Presence of EV71 antigen in spinal cords. Sections of the spinal cords (48 h p.i. into hind limb) were stained with anti-EV71 MAb (arrows) and counterstained with Nissl stain. Note that the number of Nissl stain-positive neuronal cells was diminished in the anterior horn regions (insets).
FIG. 7.
Colchicine reduces EV71 spread to the CNS. ICR mice (n = 6 to 12) were i.m. inoculated with EV71/MP4 strain (5 × 103 PFU/mouse), with colchicine treatment (12 h before inoculation), in the hind limb. Control mice received PBS. The tissue viral titer was determined at 12, 24, 36, and 48 h after viral inoculation. Results are the means and SEM. *, P < 0.05 compared with PBS-treated mice. s. c., spinal cord.
FIG. 8.
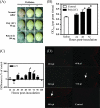
Both poly(I:C) and EV71 induce an increase in BBB permeability. ICR mice (n = 18) were i.p. injected with poly(I:C) or p.o. inoculated with EV71/MP4 (5 × 106 PFU/mouse), and the increase in BBB permeability was determined by Evans blue dye extravasation. (A) Blue discoloration of brain tissue of poly(I:C)-treated mice at 48 h p.i. (B and C) Dye extravasation was quantified by measuring the OD at 595 nm of brain homogenates from poly(I:C)-treated mice (B) and EV71-inoculated mice (C) (mean OD for control, 0.463) after extraction. Results are the means and SEM. *, P < 0.05 compared with saline control. (D) The deposition of Evans blue dye in brain tissue (arrows) was examined in frozen sections using a fluorescence microscope. Magnification, ×100.
Similar articles
- A mouse-adapted enterovirus 71 strain causes neurological disease in mice after oral infection.
Wang YF, Chou CT, Lei HY, Liu CC, Wang SM, Yan JJ, Su IJ, Wang JR, Yeh TM, Chen SH, Yu CK. Wang YF, et al. J Virol. 2004 Aug;78(15):7916-24. doi: 10.1128/JVI.78.15.7916-7924.2004. J Virol. 2004. PMID: 15254164 Free PMC article. - Pathologic characterization of a murine model of human enterovirus 71 encephalomyelitis.
Ong KC, Badmanathan M, Devi S, Leong KL, Cardosa MJ, Wong KT. Ong KC, et al. J Neuropathol Exp Neurol. 2008 Jun;67(6):532-42. doi: 10.1097/NEN.0b013e31817713e7. J Neuropathol Exp Neurol. 2008. PMID: 18520772 - Retrograde transport of intact poliovirus through the axon via the fast transport system.
Ohka S, Yang WX, Terada E, Iwasaki K, Nomoto A. Ohka S, et al. Virology. 1998 Oct 10;250(1):67-75. doi: 10.1006/viro.1998.9360. Virology. 1998. PMID: 9770421 - [Fruit of the emergence of an enterovirus: acute haemorrhagic conjunctivitis].
Sane F, Sauter P, Fronval S, Goffard A, Dewilde A, Hober D. Sane F, et al. Ann Biol Clin (Paris). 2008 Sep-Oct;66(5):485-92. doi: 10.1684/abc.2008.0257. Ann Biol Clin (Paris). 2008. PMID: 18957336 Review. French.
Cited by
- Strategies to develop antivirals against enterovirus 71.
Kuo RL, Shih SR. Kuo RL, et al. Virol J. 2013 Jan 22;10:28. doi: 10.1186/1743-422X-10-28. Virol J. 2013. PMID: 23339605 Free PMC article. Review. - Enterovirus-A71 exploits peripherin and Rac1 to invade the central nervous system.
Lim ZQ, Ng QY, Oo Y, Chu JJH, Ng SY, Sze SK, Alonso S. Lim ZQ, et al. EMBO Rep. 2021 Jun 4;22(6):e51777. doi: 10.15252/embr.202051777. Epub 2021 Apr 19. EMBO Rep. 2021. PMID: 33871166 Free PMC article. - The Current Status of the Disease Caused by Enterovirus 71 Infections: Epidemiology, Pathogenesis, Molecular Epidemiology, and Vaccine Development.
Chang PC, Chen SC, Chen KT. Chang PC, et al. Int J Environ Res Public Health. 2016 Sep 9;13(9):890. doi: 10.3390/ijerph13090890. Int J Environ Res Public Health. 2016. PMID: 27618078 Free PMC article. Review. - Inborn errors of TLR3- or MDA5-dependent type I IFN immunity in children with enterovirus rhombencephalitis.
Chen J, Jing H, Martin-Nalda A, Bastard P, Rivière JG, Liu Z, Colobran R, Lee D, Tung W, Manry J, Hasek M, Boucherit S, Lorenzo L, Rozenberg F, Aubart M, Abel L, Su HC, Soler Palacin P, Casanova JL, Zhang SY. Chen J, et al. J Exp Med. 2021 Dec 6;218(12):e20211349. doi: 10.1084/jem.20211349. Epub 2021 Nov 2. J Exp Med. 2021. PMID: 34726731 Free PMC article. - Molecular Pathogenicity of Enteroviruses Causing Neurological Disease.
Majer A, McGreevy A, Booth TF. Majer A, et al. Front Microbiol. 2020 Apr 9;11:540. doi: 10.3389/fmicb.2020.00540. eCollection 2020. Front Microbiol. 2020. PMID: 32328043 Free PMC article. Review.
References
- Bodian, D., T. Rivers, and F. Horsfall. 1959. Viral and rickettsial infections of man, p. 479-498. Lippincott, Philadelphia, PA.
- Crotty, S., L. Hix, L. J. Sigal, and R. Andino. 2002. Poliovirus pathogenesis in a new poliovirus receptor transgenic mouse model: age-dependent paralysis and a mucosal route of infection. J. Gen. Virol. 83:1707-1720. - PubMed
- Douche-Aourik, F., W. Berlier, L. Feasson, T. Bourlet, R. Harrath, S. Omar, F. Grattard, C. Denis, and B. Pozzetto. 2003. Detection of enterovirus in human skeletal muscle from patients with chronic inflammatory muscle disease or fibromyalgia and healthy subjects. J. Med. Virol. 71:540-547. - PubMed
- Ford, D. J., S. L. Ropka, G. H. Collins, and B. Jubelt. 2002. The neuropathology observed in wild-type mice inoculated with human poliovirus mirrors human paralytic poliomyelitis. Microb. Pathog. 33:97-107. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources