Detection of fractional steps in cargo movement by the collective operation of kinesin-1 motors - PubMed (original) (raw)
Detection of fractional steps in cargo movement by the collective operation of kinesin-1 motors
Cécile Leduc et al. Proc Natl Acad Sci U S A. 2007.
Abstract
The stepping behavior of single kinesin-1 motor proteins has been studied in great detail. However, in cells, these motors often do not work alone but rather function in small groups when they transport cellular cargo. Until now, the cooperative interactions between motors in such groups were poorly understood. A fundamental question is whether two or more motors that move the same cargo step in synchrony, producing the same step size as a single motor, or whether the step size of the cargo movement varies. To answer this question, we performed in vitro gliding motility assays, where microtubules coated with quantum dots were driven over a glass surface by a known number of kinesin-1 motors. The motion of individual microtubules was then tracked with nanometer precision. In the case of transport by two kinesin-1 motors, we found successive 4-nm steps, corresponding to half the step size of a single motor. Dwell-time analysis did not reveal any coordination, in the sense of alternate stepping, between the motors. When three motors interacted in collective transport, we identified distinct forward and backward jumps on the order of 10 nm. The existence of the fractional steps as well as the distinct jumps illustrate a lack of synchronization and has implications for the analysis of motor-driven organelle movement investigated in vivo.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Experimental setup of MT tracking by multicolor fluorescence microscopy. (A) Principle of an in vitro gliding motility assay. QD-coated MTs were propelled by His-tagged GFP-kinesin molecules that had been attached to the surface of a glass coverslip by Penta-His antibodies. The remainder of the surface was passivated by casein. The multicolored Tetraspeck fluorescent beads were used as references to correct for spatial drift. (B) Multicolor images of gliding motility. Rhodamine-labeled MTs were imaged by epi-fluorescence microscopy (epi-TRITC). The positions of the GFP-kinesins (laser-GFP) and the QDs (laser-QD655) were obtained by TIRF microscopy of the same field of view. The image on the right (merged) corresponds to the overlay of the three colors. The arrowheads show the kinesin positions in the laser-GFP image. The asterisk shows the appearance of two Tetraspeck beads in all colors. (Scale bar, 2 μm.)
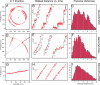
Fig. 2.
Analysis of MT motion for one, two, and many motors operating collectively. (A, D, and G) _x–y_-trajectories of individual QDs bound to a MT for the one-motor case (A), the two-motor case (D), and the multimotor case (G). The crosses in A and D mark the positions of the kinesin molecules to which the MTs are attached during swiveling. (B, E, and H) Walked distance of the tracked QDs as a function of time (red curves) for three different time intervals from the depicted x–y trajectories. In B, the walked distance was derived from the relative distance of the tracked QD from the position of the kinesin molecule. In E and H, the walked distance was derived from the projection of the QD positions onto the dashed lines in D and G, indicating the fitted pathways of the MTs during linear movement. The black lines indicate the fitted steps as obtained from the applied step-finding algorithm, and the numbers indicate the step sizes in nanometers. (C, F, and I) Histograms of pair-wise distances (_d_i − _d_j for i ≥ j) calculated from 150 consecutive data points of the curves in B, E, and H, independently from the step-finding algorithm.
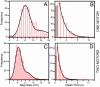
Fig. 3.
Step sizes and dwell times of the tracked QDs. (A and C) Histograms of step sizes in the one-motor case (A) and the two-motor case (C). Data were obtained from 11 different QDs in A and 17 in C. The first peaks of double-Gaussian fits (solid lines) gave step sizes of _d_1 = 8.1 ± 0.2 nm in (A) and _d_2 = 4.2 ± 0.1 nm in C. (B and D) Histograms of the corresponding dwell times. The solid lines represent the exponential fit of the dwell time distribution weighted by the square root of the count in each bin (41) (equal to the standard deviation) which gave _k_1 = 0.49 ± 0.03 s−1 (reduced χ2 = 0.4; 10 degrees of freedom) in B and _k_2 = 1.00 ± 0.04 s−1 (reduced χ2 = 1.5; 9 degrees of freedom) in D. The low numbers of events in the very first bins of the dwell-time histograms (gray shaded bins) result from the difficulty of detecting steps that are in the range of (or shorter than) the camera acquisition time. Consequently, we omitted these data in the exponential fits. We note that the number of these missed events corresponds well to the number of nonresolvable “double-steps” (16 nm for the one-motor case and 8 nm for the two-motor case) in the step-size histograms.
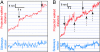
Fig. 4.
Distinct jumps in the MT displacement in the three-motor case. (A and B) Projected walked distances of two QDs (red curves) and the corresponding sideways motion (blue curves). The arrows point to the presence of jumps in the forward (solid line) and the backward (dashed line) direction. That exactly three motors were involved in transport was inferred from: (i) the multicolor fluorescent images where three green spots were in overlap with the MT and (ii) the fact that, if only two of those were active, we would expect the pronounced existence of 4-nm steps, which we did not observe.
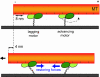
Fig. 5.
Schematic diagram of a gliding MT in the two-motor case. When one motor performs an 8-nm step, the final displacement of the QD-coated MT is 4 nm.
Similar articles
- Multiple kinesins induce tension for smooth cargo transport.
Tjioe M, Shukla S, Vaidya R, Troitskaia A, Bookwalter CS, Trybus KM, Chemla YR, Selvin PR. Tjioe M, et al. Elife. 2019 Oct 31;8:e50974. doi: 10.7554/eLife.50974. Elife. 2019. PMID: 31670658 Free PMC article. - Transport efficiency of membrane-anchored kinesin-1 motors depends on motor density and diffusivity.
Grover R, Fischer J, Schwarz FW, Walter WJ, Schwille P, Diez S. Grover R, et al. Proc Natl Acad Sci U S A. 2016 Nov 15;113(46):E7185-E7193. doi: 10.1073/pnas.1611398113. Epub 2016 Nov 1. Proc Natl Acad Sci U S A. 2016. PMID: 27803325 Free PMC article. - Studying kinesin motors by optical 3D-nanometry in gliding motility assays.
Nitzsche B, Bormuth V, Bräuer C, Howard J, Ionov L, Kerssemakers J, Korten T, Leduc C, Ruhnow F, Diez S. Nitzsche B, et al. Methods Cell Biol. 2010;95:247-71. doi: 10.1016/S0091-679X(10)95014-0. Methods Cell Biol. 2010. PMID: 20466139 Review. - Processive movement of single kinesins on crowded microtubules visualized using quantum dots.
Seitz A, Surrey T. Seitz A, et al. EMBO J. 2006 Jan 25;25(2):267-77. doi: 10.1038/sj.emboj.7600937. Epub 2006 Jan 12. EMBO J. 2006. PMID: 16407972 Free PMC article. - Bidirectional transport of organelles: unity and struggle of opposing motors.
Bryantseva SA, Zhapparova ON. Bryantseva SA, et al. Cell Biol Int. 2012 Jan;36(1):1-6. doi: 10.1042/CBI20110413. Cell Biol Int. 2012. PMID: 22142363 Review.
Cited by
- The Tail of Kinesin-14a in Giardia Is a Dual Regulator of Motility.
Tseng KF, Mickolajczyk KJ, Feng G, Feng Q, Kwok ES, Howe J, Barbar EJ, Dawson SC, Hancock WO, Qiu W. Tseng KF, et al. Curr Biol. 2020 Sep 21;30(18):3664-3671.e4. doi: 10.1016/j.cub.2020.06.090. Epub 2020 Jul 30. Curr Biol. 2020. PMID: 32735815 Free PMC article. - Wave-like oscillations of clamped microtubules driven by collective dynein transport.
Yadav SA, Khatri D, Soni A, Khetan N, Athale CA. Yadav SA, et al. Biophys J. 2024 Feb 20;123(4):509-524. doi: 10.1016/j.bpj.2024.01.016. Epub 2024 Jan 22. Biophys J. 2024. PMID: 38258292 Free PMC article. - Myo4p is a monomeric myosin with motility uniquely adapted to transport mRNA.
Dunn BD, Sakamoto T, Hong MS, Sellers JR, Takizawa PA. Dunn BD, et al. J Cell Biol. 2007 Sep 24;178(7):1193-206. doi: 10.1083/jcb.200707080. J Cell Biol. 2007. PMID: 17893244 Free PMC article. - Motor number controls cargo switching at actin-microtubule intersections in vitro.
Schroeder HW 3rd, Mitchell C, Shuman H, Holzbaur EL, Goldman YE. Schroeder HW 3rd, et al. Curr Biol. 2010 Apr 27;20(8):687-96. doi: 10.1016/j.cub.2010.03.024. Epub 2010 Apr 15. Curr Biol. 2010. PMID: 20399098 Free PMC article. - Challenges in Estimating the Motility Parameters of Single Processive Motor Proteins.
Ruhnow F, Kloβ L, Diez S. Ruhnow F, et al. Biophys J. 2017 Dec 5;113(11):2433-2443. doi: 10.1016/j.bpj.2017.09.024. Biophys J. 2017. PMID: 29211997 Free PMC article.
References
- Howard J. Mechanics of Motor Proteins and the Cytoskeleton. Sunderland, MA: Sinauer; 2001.
- Carter NJ, Cross RA. Curr Opin Cell Biol. 2006;18:61–67. - PubMed
- Sellers JR, Veigel C. Curr Opin Cell Biol. 2006;18:68–73. - PubMed
- Howard J, Hudspeth AJ, Vale RD. Nature. 1989;342:154–158. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources