Protein kinase C iota: human oncogene, prognostic marker and therapeutic target - PubMed (original) (raw)
Review
Protein kinase C iota: human oncogene, prognostic marker and therapeutic target
Alan P Fields et al. Pharmacol Res. 2007 Jun.
Abstract
The protein kinase C (PKC) family of serine/threonine kinases has been the subject of intensive study in the field of cancer since their initial discovery as major cellular receptors for the tumor promoting phorbol esters nearly 30 years ago. However, despite these efforts, the search for a direct genetic link between members of the PKC family and human cancer has yielded only circumstantial evidence that any PKC isozyme is a true cancer gene. This situation changed in the past year with the discovery that atypical protein kinase C iota (PKC iota) is a bonafide human oncogene. PKC iota is required for the transformed growth of human cancer cells and the PKC iota gene is the target of tumor-specific gene amplification in multiple forms of human cancer. PKC iota participates in multiple aspects of the transformed phenotype of human cancer cells including transformed growth, invasion and survival. Herein, we review pertinent aspects of atypical PKC structure, function and regulation that relate to the role of these enzymes in oncogenesis. We discuss the evidence that PKC iota is a human oncogene, review mechanisms controlling PKC iota expression in human cancers, and describe the molecular details of PKC iota-mediated oncogenic signaling. We conclude with a discussion of how oncogenic PKC iota signaling has been successfully targeted to identify a novel, mechanism-based therapeutic drug currently entering clinical trials for treatment of human lung cancer. Throughout, we identify key unanswered questions and exciting future avenues of investigation regarding this important oncogenic molecule.
Figures
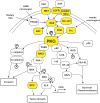
Figure 1. Schematic of major oncogenic PKCι signaling pathways
PKCι resides in several major signaling pathways implicated in human cancer. Many components of these pathway are mutated, often by multiple mechanisms (ie. gene amplification and somatic mutation), in human tumors (indicated by yellow boxes). Arrows indicate flow through signaling pathways; touching boxes indicate directly binding of signaling components. Phosphorylation events are indicated by circled Ps.
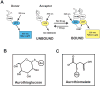
Figure 2. Schematic diagram illustrating the basis of a FRET-based assay to screen for inhibitors of the Par6/PKCι interaction
(A) When exposed to 395 nm light, the donor molecule Par6/CFP, emits blue fluorescent light with an emission maximum at 475 nm. The acceptor molecule PKCι/YFP does not fluoresce when exposed to 395 nm light. When Par6/CFP and PKCι/YFP are bound, excitation of the donor with 395 nm light leads not only to emission of blue light at 475 nm, but also to FRET-mediated emission of yellow light at 529 nm. In the presence of a chemical inhibitor such as ATG or ATM, FRET is blocked. Chemical structures of ATG (B) and ATM (C), respectively.
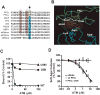
Figure 3. ATM targets Cys 69 on PKCι to inhibit PKCι-par6 binding and PKCι-dependent transformed growth
(A) Sequence alignment of the OPCA motifs of 10 human PB1 domain-containing proteins reveals that cysteine 69 (Cys69) is unique to the OPCA motif of PKCι and PKCζ. (B) The crystal structure of the PKCι-Par6complex reveals that Cys69 of PKCι resides in the binding interface between PKCι (green) and Par6 (blue) where it interacts with Arg28 on Par6. (C) Mutation of Cys69 on PKCι to isoleucine or valine abolishes the inhibitory effect of ATM on Par6 binding. (D) NSCLC cells stably expressing a PKCι C69I mutant are resistant to the inhibitory effects of ATM on transformed growth.
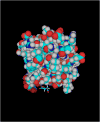
Figure 4. Molecular model of the PKCι-Cys69-ATM adduct based on the crystal structure of the PKCι PB1 domain
The Cys69-ATM adduct protrudes into the binding cleft normally occupied by Par6 in the PKCι-Par6 complex (bottom of structure).
Similar articles
- Atypical protein kinase C iota expression and aurothiomalate sensitivity in human lung cancer cells.
Regala RP, Thompson EA, Fields AP. Regala RP, et al. Cancer Res. 2008 Jul 15;68(14):5888-95. doi: 10.1158/0008-5472.CAN-08-0438. Cancer Res. 2008. PMID: 18632643 Free PMC article. - Atypical protein kinase C iota is an oncogene in human non-small cell lung cancer.
Regala RP, Weems C, Jamieson L, Khoor A, Edell ES, Lohse CM, Fields AP. Regala RP, et al. Cancer Res. 2005 Oct 1;65(19):8905-11. doi: 10.1158/0008-5472.CAN-05-2372. Cancer Res. 2005. PMID: 16204062 - Atypical protein kinase C{iota} is required for bronchioalveolar stem cell expansion and lung tumorigenesis.
Regala RP, Davis RK, Kunz A, Khoor A, Leitges M, Fields AP. Regala RP, et al. Cancer Res. 2009 Oct 1;69(19):7603-11. doi: 10.1158/0008-5472.CAN-09-2066. Epub 2009 Sep 8. Cancer Res. 2009. PMID: 19738040 Free PMC article. - Targeting the oncogenic protein kinase Ciota signalling pathway for the treatment of cancer.
Fields AP, Frederick LA, Regala RP. Fields AP, et al. Biochem Soc Trans. 2007 Nov;35(Pt 5):996-1000. doi: 10.1042/BST0350996. Biochem Soc Trans. 2007. PMID: 17956262 Review. - Nonmutagenic mechanisms in carcinogenesis: role of protein kinase C in signal transduction and growth control.
Weinstein IB. Weinstein IB. Environ Health Perspect. 1991 Jun;93:175-9. doi: 10.1289/ehp.9193175. Environ Health Perspect. 1991. PMID: 1773790 Free PMC article. Review.
Cited by
- Wealth of opportunity - the C1 domain as a target for drug development.
Blumberg PM, Kedei N, Lewin NE, Yang D, Czifra G, Pu Y, Peach ML, Marquez VE. Blumberg PM, et al. Curr Drug Targets. 2008 Aug;9(8):641-52. doi: 10.2174/138945008785132376. Curr Drug Targets. 2008. PMID: 18691011 Free PMC article. Review. - Par complex in cancer: a regulator of normal cell polarity joins the dark side.
Aranda V, Nolan ME, Muthuswamy SK. Aranda V, et al. Oncogene. 2008 Nov 24;27(55):6878-87. doi: 10.1038/onc.2008.340. Oncogene. 2008. PMID: 19029931 Free PMC article. Review. - Protein kinase C-iota-mediated glycolysis promotes non-small-cell lung cancer progression.
Liu L, Lei B, Wang L, Chang C, Yang H, Liu J, Huang G, Xie W. Liu L, et al. Onco Targets Ther. 2019 Jul 18;12:5835-5848. doi: 10.2147/OTT.S207211. eCollection 2019. Onco Targets Ther. 2019. PMID: 31410027 Free PMC article. - Investigation of UTR Variants by Computational Approaches Reveal Their Functional Significance in PRKCI Gene Regulation.
Shah H, Khan K, Badshah Y, Mahmood Ashraf N, Shabbir M, Trembley JH, Afsar T, Abusharha A, Razak S. Shah H, et al. Genes (Basel). 2023 Jan 18;14(2):247. doi: 10.3390/genes14020247. Genes (Basel). 2023. PMID: 36833174 Free PMC article. - Protein kinase C iota promotes glycolysis via PI3K/AKT/mTOR signalling in high grade serous ovarian cancer.
Tyagi K, Roy A, Mandal S. Tyagi K, et al. Mol Biol Rep. 2024 Sep 14;51(1):983. doi: 10.1007/s11033-024-09918-3. Mol Biol Rep. 2024. PMID: 39276277
References
- Takai Y, Yamamoto M, Inoue M, Kishimoto A, Nishizuka Y. A proenzyme of cyclic nucleotide-independent protein kinase and its activation by calcium-dependent neutral protease from rat liver. Biochem Biophys Res Commun. 1977;77(2):542–50. - PubMed
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–14. - PubMed
- Nishizuka Y. The Albert Lasker Medical Awards. The family of protein kinase C for signal transduction. Jama. 1989;262(13):1826–33. - PubMed
- Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. Faseb J. 1995;9(7):484–96. - PubMed
- Kikkawa U, Kishimoto A, Nishizuka Y. The protein kinase C family: heterogeneity and its implications. Annu Rev Biochem. 1989;58:31–44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous