Can biomarkers identify women at increased stroke risk? The Women's Health Initiative Hormone Trials - PubMed (original) (raw)
Can biomarkers identify women at increased stroke risk? The Women's Health Initiative Hormone Trials
Charles Kooperberg et al. PLoS Clin Trials. 2007.
Abstract
Objective: The Women's Health Initiative hormone trials identified a 44% increase in ischemic stroke risk with combination estrogen plus progestin and a 39% increase with estrogen alone. We undertook a case-control biomarker study to elucidate underlying mechanisms, and to potentially identify women who would be at lower or higher risk for stroke with postmenopausal hormone therapy (HT).
Design: The hormone trials were randomized, double-blind, and placebo controlled.
Setting: The Women's Health Initiative trials were conducted at 40 clinical centers in the United States.
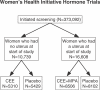
Participants: The trials enrolled 27,347 postmenopausal women, aged 50-79 y.
Interventions: We randomized 16,608 women with intact uterus to conjugated estrogens 0.625 mg with medroxyprogesterone acetate 2.5 mg daily or placebo, and 10,739 women with prior hysterectomy to conjugated estrogens 0.625 mg daily or placebo.
Outcome measures: Stroke was ascertained during 5.6 y of follow-up in the estrogen plus progestin trial and 6.8 y of follow-up in the estrogen alone trial.
Results: No baseline clinical characteristics, including gene polymorphisms, identified women for whom the stroke risk from HT was higher. Paradoxically, women with higher baseline levels of some stroke-associated biomarkers had a lower risk of stroke when assigned to estrogen plus progestin compared to placebo. For example, those with higher IL-6 were not at increased stroke risk when assigned to estrogen plus progestin (odds ratio 1.28) but were when assigned to placebo (odds ratio 3.47; p for difference = 0.02). Similar findings occurred for high baseline PAP, leukocyte count, and D-dimer. However, only an interaction of D-dimer during follow-up interaction with HT and stroke was marginally significant (p = 0.03).
Conclusions: Biomarkers did not identify women at higher stroke risk with postmenopausal HT. Some biomarkers appeared to identify women at lower stroke risk with estrogen plus progestin, but these findings may be due to chance.
Conflict of interest statement
Competing Interests MC participated in three meetings as a consultant for Pharmacia, Novartis, and Merck on topics related to hormone therapies or selective estrogen receptor modulators in 2001 and 2003. JGR has received to date: Grants from Abbott, Andrx Labs, Astra-Zeneca, Atherogenics, Bristol-Myers Squibb, GlaxoSmithKline, Hoffman La Roche, Merck, Pfizer, Procter & Gamble, Sankyo, Schering-Plough, Takeda, and Wyeth Ayerst; Speaker honoraria for education programs from Bristol-Myers Squibb, Merck, and Pfizer; Honoraria from Reliant; Consultant/Advisory Board for Bristol -Myers Squibb, Merck, Pfizer, Proliant, Wellmark, and American Emu Association.
Figures
Figure 1. Women's Health Initiative Hormone Trials
CEE, conjugated equine estrogens; MPA, medroxyprogestrone acetate.
Similar articles
- Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial.
Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner RL, Manson JE, Gass ML, Stefanick ML, Lane DS, Hays J, Johnson KC, Coker LH, Dailey M, Bowen D; WHIMS Investigators. Rapp SR, et al. JAMA. 2003 May 28;289(20):2663-72. doi: 10.1001/jama.289.20.2663. JAMA. 2003. PMID: 12771113 Clinical Trial. - Effect of estrogen plus progestin on stroke in postmenopausal women: the Women's Health Initiative: a randomized trial.
Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, Kotchen T, Curb JD, Black H, Rossouw JE, Aragaki A, Safford M, Stein E, Laowattana S, Mysiw WJ; WHI Investigators. Wassertheil-Smoller S, et al. JAMA. 2003 May 28;289(20):2673-84. doi: 10.1001/jama.289.20.2673. JAMA. 2003. PMID: 12771114 Clinical Trial. - Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study.
Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH; Women's Health Initiative Memory Study. Shumaker SA, et al. JAMA. 2004 Jun 23;291(24):2947-58. doi: 10.1001/jama.291.24.2947. JAMA. 2004. PMID: 15213206 Clinical Trial. - The Women's Health Initiative trial and related studies: 10 years later: a clinician's view.
Gurney EP, Nachtigall MJ, Nachtigall LE, Naftolin F. Gurney EP, et al. J Steroid Biochem Mol Biol. 2014 Jul;142:4-11. doi: 10.1016/j.jsbmb.2013.10.009. Epub 2013 Oct 27. J Steroid Biochem Mol Biol. 2014. PMID: 24172877 Review. - A comparative review of the risks and benefits of hormone replacement therapy regimens.
Warren MP. Warren MP. Am J Obstet Gynecol. 2004 Apr;190(4):1141-67. doi: 10.1016/j.ajog.2003.09.033. Am J Obstet Gynecol. 2004. PMID: 15118656 Review.
Cited by
- Postmenopausal estrogen and progestin effects on the serum proteome.
Pitteri SJ, Hanash SM, Aragaki A, Amon LM, Chen L, Busald Buson T, Paczesny S, Katayama H, Wang H, Johnson MM, Zhang Q, McIntosh M, Wang P, Kooperberg C, Rossouw JE, Jackson RD, Manson JE, Hsia J, Liu S, Martin L, Prentice RL. Pitteri SJ, et al. Genome Med. 2009 Dec 24;1(12):121. doi: 10.1186/gm121. Genome Med. 2009. PMID: 20034393 Free PMC article. - Postmenopausal hormone therapy and the risks of coronary heart disease, breast cancer, and stroke.
Prentice RL. Prentice RL. Semin Reprod Med. 2014 Nov;32(6):419-25. doi: 10.1055/s-0034-1384624. Epub 2014 Oct 16. Semin Reprod Med. 2014. PMID: 25321418 Free PMC article. Review. - The cross-sectional association between vasomotor symptoms and hemostatic parameter levels in postmenopausal women.
Harrington LB, Blondon M, Cushman M, Kaunitz AM, Rossouw JE, Allison MA, Martin LW, Johnson KC, Rosing J, Woods NF, LaCroix AZ, Heckbert SR, McKnight B, Smith NL. Harrington LB, et al. Menopause. 2017 Apr;24(4):360-370. doi: 10.1097/GME.0000000000000777. Menopause. 2017. PMID: 27922933 Free PMC article. - Association of Serum Sex Hormones with Hemostatic Factors in Women On and Off Hormone Therapy: The Multiethnic Study of Atherosclerosis.
Williams MS, Cushman M, Ouyang P, Heckbert SR, Kalyani RR, Vaidya D. Williams MS, et al. J Womens Health (Larchmt). 2016 Feb;25(2):166-72. doi: 10.1089/jwh.2015.5465. Epub 2015 Dec 24. J Womens Health (Larchmt). 2016. PMID: 26700933 Free PMC article. - Bone Turnover Markers Are Not Associated With Hip Fracture Risk: A Case-Control Study in the Women's Health Initiative.
Crandall CJ, Vasan S, LaCroix A, LeBoff MS, Cauley JA, Robbins JA, Jackson RD, Bauer DC. Crandall CJ, et al. J Bone Miner Res. 2018 Jul;33(7):1199-1208. doi: 10.1002/jbmr.3471. Epub 2018 Jun 19. J Bone Miner Res. 2018. PMID: 29923225 Free PMC article.
References
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. - PubMed
- Anderson GL, Limacher M, Assaf AR, Bassford SA, Black H, et al. Effects of conjugated equine estrogen on postmenopausal women with hysterectomy: The Women's Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. - PubMed
- Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, et al. Effect of estrogen plus progestin on stroke in postmenopausal women: The Women's Health Initiative: A randomized trial. JAMA. 2003;289:2673–2684. - PubMed
- Hendrix SL, Wassertheil-Smoller S, Johnson KC, Howard BV, Kooperberg C, et al. Effects of conjugated equine estrogen on stroke in the Women's Health Initiative. Circulation. 2006;113:2425–2434. - PubMed
- Women's Health Initiative Study Group. Design of the Women's Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19:61–109. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials