Role of CD151, A tetraspanin, in porcine reproductive and respiratory syndrome virus infection - PubMed (original) (raw)
Role of CD151, A tetraspanin, in porcine reproductive and respiratory syndrome virus infection
Kumar Shanmukhappa et al. Virol J. 2007.
Abstract
Background: Porcine reproductive and respiratory syndrome virus (PRRSV) is a RNA virus causing respiratory and reproductive diseases in swine. The susceptibility for PRRSV varies between the different breeds of swine. In cell culture, PRRSV virus can be propagated in primary porcine alveolar macrophages and some African green monkey kidney cell lines, such as MARC-145 cells. Previous studies have shown that 3' untranslated region (UTR) RNAs of the arteriviruses play an important role in the replication of the virus through interactions with cellular proteins. To better understand the differences in the replication capability of PRRSV in different cell lines, we sought to identify the host cellular proteins interacting with PRRSV 3' UTR RNA. We constructed a cDNA library of MARC-145 cell line in lambda ZAP Express vector and screened the library with the positive sense 3' UTR RNA of PRRSV.
Results: We found that CD151, a host cellular protein, interacting with PRRSV 3' UTR RNA. The specificity of the interaction between CD151 and PRRSV 3' UTR RNA was examined by gel shift assay as well as North-Western hybridization. The transfection of CD151 expression clone into BHK-21 rendered these cells susceptible to PRRSV infection, and the transfection of siRNA against CD151 into MARC-145 significantly reduced the level of PRRSV infection. Also, anti-CD151 antibody treatment to MARC-145 completely blocked PRRSV infection.
Conclusion: Based on our results, we suggest that CD151 should cooperate in PRRSV infection in vitro in MARC-145 and BHK-21 cells.
Figures
Figure 1
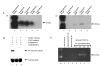
Alignment of CD151 amino acid sequences. Simian CD151 amino acid sequence was generated from the cDNA sequence. The amino acid sequence was aligned with human, bovine, murine and porcine CD151 amino acid sequences. Dots represent similarity of amino acid residues. Genbank accession number is AF 275666 [Genbank: AF275666].
Figure 2
RNA-binding activity of CD151 in vitro and in vivo. (A) In vito RNA-binding activity of CD151 was demonstrated by Immunoprecipitation/North-Western blot analysis. BHK-21 and MARC-145 cells were transfected with pBK-CMV plasmid expressing CD 151 as a β-galactosidase fusion protein. The cell lysates were immunoprecipitated with anti-CD151 MAb (A1) and anti-β-galactosidase MAb (A2). In A1, lane1, MARC-145 cytoplasmic protein lysate (without immunoprecipitation); lanes 2, transfected MARC-145; lane 3, transfected BHK-21; lane 4, untransfected MARC-145; lane 5, untransfected BHK-21. In A (2), lane1, MARC-145 cytoplasmic protein lysate (without immunoprecipitation); lane 2, untransfected BHK-21; lane 3, transfected BHK-21; lane 4, transfected MARC-145; lane 5, untransfected MARC-145. FIG 2B, gel shift assay demonstrating the interaction of CD151 protein with the PRRSV 3'UTR RNA. MARC cell lysate was immunoprecipitated with CD151 antibody (lanes 1&3) and the complex was incubated radiolabelled PRRSV 3' UTR RNA. Addition of unlabelled RNA (lane 3) prevented the formation of complex, while the radiolabelled RNA did not interact with the CD151 antibody (lane 3). FIG 2C, In vivo RNA-binding activity of CD151 was demonstrated by immunoprecipitation/RT-PCR assay (149 bp amplicon). PRRSV-infected or uninfected MARC-145 cell lysates were immunoprecipitated with anti-CD151 MAb or a negative control MAb (wasp, Cotesia folepis MAb), and RT-PCR was performed using PRRSV 3' UTR RNA-specific primers for RNAs extracted from the immunocomplexes. M, 123 bp ladder; lane 1, negative PCR control; lane 2, PRRSV-uninfected/CD151 MAb-immunoprecipitated; lane 3, PRRSV-infected/wasp MAb-immunoprecipitated; lane 4, PRRSV-infected/CD151 MAb-immunoprecipitated (without UV cross-linking); lane 5, PRRSV-infected/CD151 MAb-immunoprecipitated (UV cross-linked for 15 min); lane 6, PRRSV-infected/CD151 MAb-immunoprecipitated (UV cross-linked for 30 min); lane 7, PRRSV-infected/CD151 MAb-immunoprecipitated (UV cross-linked for 45 min).
Figure 3
Detection of the presence of CD151 by RT-PCR and Western blot. Correlation between CD151 expression and susceptibility to PRRSV infection was demonstrated by RT-PCR and Western blot analysis. (A) RT-PCR showing the amplification of 105 bp amplicon with CD151-specific primers was performed for RNAs isolated from PRRSV-susceptible and -non-susceptible cell lines. M, 123 bp ladder; lane 1, negative RT control; lane 2, negative PCR control; lane 3, HRT; lane 4, MARC-145; lane 5, MDBK; lane 6, BHK-21; lane 7, ST; lane 8, MA-104; lane 9, ST-K; lane 10, Vero; lane 11, CL-2621; lane 12, COS; lane 13, CD151-transfected BHK-21. (B) Western blot analysis using anti-CD151 MAb was performed for cell lysates from PRRSV-susceptible and -non susceptible cell lines. Lane 1, MARC-145; lane 2, BHK-21; lane 3, Vero. (C) Flow cytometric analysis using polyclonal anti-CD151 Ab was performed for MARC-145 (C (1)) and BHK-21 (C (2)) cell lines. An isotype-matched control is represented by the dotted lines.
Figure 4
Transfection of simian CD151 into BHK-21 cells. To detect the presence of PRRSV in simian CD151-transfected BHK-21 cells, immunohistochemical staining was performed using SR-30, a MAb against PRRSV nucleocapsid protein. (A) Simian CD151-untransfected BHK-21 cells, and (B) Simian CD151-transfected BHK-21 cells. The presence of PRRSV is shown by DAB substrate in brown color.
Figure 5
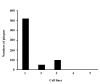
Effect of CD151-overexpression on PRRSV infection. The effect of CD151-overexpression on PRRSV infection was demonstrated by virus burst assay. To induce CD151-overexpression, the simian CD151 expressing clone was transfected into MARC-145 cells. Column 1, CD151-transfected/PRRSV-infected MARC-145; column 2, β-galactosidase-transfected/PRRSV-infected MARC-145; column 3, CD151-transfected/PRRSV-infected BHK-21; column 4, CD151-untransfected/PRRSV-infected BHK-21; column 5, CD151-transfected/PRRSV-uninfected MARC.
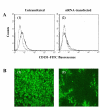
Figure 6
Effect of siRNA against CD151 on PRRSV infection. (A) To examine the effect of siRNA against CD151 on PRRSV infection, siRNA was transfected into MARC-145 cells. The suppression of the cell surface expression of CD151 by the transfection of siRNA was shown by flow cytometric analysis for the untransfected MARC-145 cells (A1) and the transfected MARC-145 cells (A2). An isotype-matched control is represented by the dotted lines. (B) The effect of siRNA on PRRSV infection was shown by immunofluorescence antibody assay using FITC-conjugated SDOW-17, a MAb against PRRSV nucleocapsid protein for the untransfected MARC-145 cells (B 1) and the transfected MARC-145 cells (B 2).
Figure 7
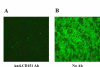
Effect of anti-CD151 Ab on PRRSV infection. To examine the effect of anti-CD151 Ab on PRRSV infection, immunofluorescence antibody assay was performed. MARC-145 cells were incubated with polyclonal anti-CD151 Ab (A) or PBS (B) and infected with PRRSV. At 2 days post infection, the presence of PRRSV in the cells was detected by FITC-conjugated SDOW-17, a MAb against PRRSV nucleocapsid protein.
Similar articles
- Interference of porcine reproductive and respiratory syndrome virus replication on MARC-145 cells using DNA-based short interfering RNAs.
He YX, Hua RH, Zhou YJ, Qiu HJ, Tong GZ. He YX, et al. Antiviral Res. 2007 May;74(2):83-91. doi: 10.1016/j.antiviral.2006.04.013. Epub 2006 May 11. Antiviral Res. 2007. PMID: 16730075 - Construction and evaluation of genetically engineered replication-defective porcine reproductive and respiratory syndrome virus vaccine candidates.
Welch SK, Jolie R, Pearce DS, Koertje WD, Fuog E, Shields SL, Yoo D, Calvert JG. Welch SK, et al. Vet Immunol Immunopathol. 2004 Dec 8;102(3):277-90. doi: 10.1016/j.vetimm.2004.09.022. Vet Immunol Immunopathol. 2004. PMID: 15507311 Clinical Trial. - Inhibition of porcine reproductive and respiratory syndrome virus replication by short hairpin RNA in MARC-145 cells.
Huang J, Jiang P, Li Y, Xu J, Jiang W, Wang X. Huang J, et al. Vet Microbiol. 2006 Jul 20;115(4):302-10. doi: 10.1016/j.vetmic.2006.02.018. Epub 2006 Apr 11. Vet Microbiol. 2006. PMID: 16584853 - Apoptosis and porcine reproductive and respiratory syndrome virus.
Miller LC, Fox JM. Miller LC, et al. Vet Immunol Immunopathol. 2004 Dec 8;102(3):131-42. doi: 10.1016/j.vetimm.2004.09.004. Vet Immunol Immunopathol. 2004. PMID: 15507300 Review. - PRRSV, the virus.
Meulenberg JJ. Meulenberg JJ. Vet Res. 2000 Jan-Feb;31(1):11-21. doi: 10.1051/vetres:2000103. Vet Res. 2000. PMID: 10726635 Review.
Cited by
- Developing a Triple Transgenic Cell Line for High-Efficiency Porcine Reproductive and Respiratory Syndrome Virus Infection.
Zhang L, Cui Z, Zhou L, Kang Y, Li L, Li J, Dai Y, Yu S, Li N. Zhang L, et al. PLoS One. 2016 May 16;11(5):e0154238. doi: 10.1371/journal.pone.0154238. eCollection 2016. PLoS One. 2016. PMID: 27182980 Free PMC article. - Key Gaps in the Knowledge of the Porcine Respiratory Reproductive Syndrome Virus (PRRSV).
Montaner-Tarbes S, Del Portillo HA, Montoya M, Fraile L. Montaner-Tarbes S, et al. Front Vet Sci. 2019 Feb 20;6:38. doi: 10.3389/fvets.2019.00038. eCollection 2019. Front Vet Sci. 2019. PMID: 30842948 Free PMC article. Review. - Establishment and Characterization of a High and Stable Porcine CD163-Expressing MARC-145 Cell Line.
Wu X, Qi J, Cong X, Chen L, Hu Y, Yoo D, Wang G, Tian F, Li F, Sun W, Chen Z, Guo L, Wu J, Li J, Wang J, Zhao X, Du Y. Wu X, et al. Biomed Res Int. 2018 Feb 21;2018:4315861. doi: 10.1155/2018/4315861. eCollection 2018. Biomed Res Int. 2018. PMID: 29682543 Free PMC article. - The Function of the PRRSV-Host Interactions and Their Effects on Viral Replication and Propagation in Antiviral Strategies.
Ma J, Ma L, Yang M, Wu W, Feng W, Chen Z. Ma J, et al. Vaccines (Basel). 2021 Apr 9;9(4):364. doi: 10.3390/vaccines9040364. Vaccines (Basel). 2021. PMID: 33918746 Free PMC article. Review. - Identification and biophysical characterization of epitope atlas of Porcine Reproductive and Respiratory Syndrome Virus.
Dey S, Bruner J, Brown M, Roof M, Chowdhury R. Dey S, et al. Comput Struct Biotechnol J. 2024 Sep 10;23:3348-3357. doi: 10.1016/j.csbj.2024.08.029. eCollection 2024 Dec. Comput Struct Biotechnol J. 2024. PMID: 39310279 Free PMC article.
References
- Bautista EM, Goyal SM, Collins JE. Serologic survey for Lelystad and VR-2332 strains of porcine respiratory and reproductive syndrome (PRRS) virus in US swine herds. J Vet Diagn Invest. 1993;5:612–614. - PubMed
- Bautista EM, Goyal SM, Yoon IJ, Joo HS, Collins JE. Comparison of porcine alveolar macrophages and CL 2621 for the detection of porcine reproductive and respiratory syndrome (PRRS) virus and anti-PRRS antibody. J Vet Diagn Invest. 1993;5:163–165. - PubMed
- Goyal SM. Porcine reproductive and respiratory syndrome. J Vet Diagn Invest. 1993;5:656–664. - PubMed
- Meulenberg JJ, de Meijer EJ, Moormann RJ. Subgenomic RNAs of Lelystad virus contain a conserved leader-body junction sequence. J Gen Virol. 1993;74:1697–1701. - PubMed
- Meulenberg JJ, Hulst MM, de Meijer EJ, Moonen PL, den Besten A, de Kluyver EP, Wensvoort G, Moormann RJ. Lelystad virus, the causative agent of porcine epidemic abortion and respiratory syndrome (PEARS), is related to LDV and EAV. Virology. 1993;192:62–72. doi: 10.1006/viro.1993.1008. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources