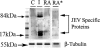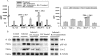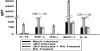Antiviral and anti-inflammatory effects of rosmarinic acid in an experimental murine model of Japanese encephalitis - PubMed (original) (raw)
Antiviral and anti-inflammatory effects of rosmarinic acid in an experimental murine model of Japanese encephalitis
Vivek Swarup et al. Antimicrob Agents Chemother. 2007 Sep.
Abstract
Rosmarinic acid (RA) reduced the mortality of mice infected with Japanese encephalitis virus (JEV). Significant decreases in viral loads (P < 0.001) and proinflammatory cytokine levels (P < 0.001) were observed in JEV-infected animals treated with RA compared to levels in infected mice without treatment, at 8 to 9 days postinfection.
Figures
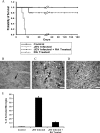
FIG. 1.
RA treatment significantly increases the survival of JEV-infected mice. (A) Survival of mice infected with 3 × 105 PFU of JEV was significantly increased in groups that received RA treatment (15 mice for each group). Treatment with RA alone has no significant effect on survival. Observation of animal survival experiments was performed in a masked manner to avoid bias toward any one group of animals. (B to E) Cryostat sections from control (B), JEV-infected (C), and JEV-infected and RA-treated (D) mouse brains were processed for Iba-1. While the control sections exhibited only resting microglia (B; arrow), the infected brains showed the presence of activated microglia (C; arrowheads). The group of JEV-infected and RA-treated mice had mostly resting microglia (D; arrow). Scale bar, 25 μm. (E) Iba-1-positive activated microglia were counted and plotted as a graph. Values represent the means ± standard errors of the means (SEM) from five random fields in three animals in each group (P < 0.001).
FIG. 2.
Antiviral efficacy of RA. C, control mice; I, JEV-infected mice; RA, JEV-infected and RA-treated mice; RA*, JEV-infected and RA-treated mice (dead). RA treatment in JEV-infected mice completely reduced the levels of viral proteins. Proteins isolated from control, JEV-infected, and JEV-infected and RA-treated BALB/c mice were analyzed by immunoblot analysis. A significant decrease in the levels of JEV-specific proteins (84 kDa and 17 kDa) was observed in RA-treated samples compared to levels in infected mice. Data shown are representative of four individual animals from each group.
FIG. 3.
RA abrogates the increased expression of proinflammatory mediators. (A) Expression of IL-12, TNF-α, IFN-γ, MCP-1, and IL-6 was observed by CBA in control animals, JEV-infected animals, JEV-infected and RA-treated animals, animals treated with RA alone, and JEV-infected and RA-treated animals at death. Levels of IL-12, TNF-α, IFN-γ, MCP-1, and IL-6 were significantly reduced in RA-treated samples compared to levels in infected mice. P < 0.001 (mean ± SEM). JEV-infected mice that succumbed even after RA treatment had significantly high levels of proinflammatory cytokines compared to uninfected mice. P < 0.001 (mean ± SEM) (four mice for each group). (B) Protein levels of control, JEV-infected, JEV-infected and RA-treated, RA-treated, and JEV-infected and RA-treated animals at death were analyzed by immunoblotting. Significant reductions in the levels of pNF-κB and Cox-2 in RA-treated samples were observed compared to levels in infected samples (P < 0.05). RA treatment reversed the level of IκB-α (P < 0.05). Mice that died even after RA treatment had increased levels of pNF-κB and Cox-2 and decreased levels of IκB-α compared to the control group. Data shown are for two individual animals from a total of four animals in each group.
FIG. 4.
RA decreases the proinflammatory mediators in vitro. Mouse microglial cell line BV-2 was used to study the in vitro induction of proinflammatory cytokines and a chemokine following JEV infection. There were significant reductions in the levels of IL-6, MCP-1, IL-12, IFN-γ, and TNF-α in JEV-infected and RA-treated samples compared to levels in JEV-infected samples. Values represent the means ± SEM from three independent experiments performed in duplicate (P < 0.05).
Similar articles
- Modulation of the immune-related gene responses to protect mice against Japanese encephalitis virus using the antimicrobial peptide, tilapia hepcidin 1-5.
Huang HN, Rajanbabu V, Pan CY, Chan YL, Hui CF, Chen JY, Wu CJ. Huang HN, et al. Biomaterials. 2011 Oct;32(28):6804-14. doi: 10.1016/j.biomaterials.2011.05.053. Epub 2011 Jul 2. Biomaterials. 2011. PMID: 21726898 - Minocycline neuroprotects, reduces microglial activation, inhibits caspase 3 induction, and viral replication following Japanese encephalitis.
Mishra MK, Basu A. Mishra MK, et al. J Neurochem. 2008 Jun;105(5):1582-95. doi: 10.1111/j.1471-4159.2008.05238.x. Epub 2008 Jan 18. J Neurochem. 2008. PMID: 18208541 - Therapeutic effect of a novel anilidoquinoline derivative, 2-(2-methyl-quinoline-4ylamino)-N-(2-chlorophenyl)-acetamide, in Japanese encephalitis: correlation with in vitro neuroprotection.
Ghosh J, Swarup V, Saxena A, Das S, Hazra A, Paira P, Banerjee S, Mondal NB, Basu A. Ghosh J, et al. Int J Antimicrob Agents. 2008 Oct;32(4):349-54. doi: 10.1016/j.ijantimicag.2008.05.001. Epub 2008 Jul 31. Int J Antimicrob Agents. 2008. PMID: 18674886 - Antioxidants: potential antiviral agents for Japanese encephalitis virus infection.
Zhang Y, Wang Z, Chen H, Chen Z, Tian Y. Zhang Y, et al. Int J Infect Dis. 2014 Jul;24:30-6. doi: 10.1016/j.ijid.2014.02.011. Epub 2014 Apr 26. Int J Infect Dis. 2014. PMID: 24780919 Review. - Japanese encephalitis revisited.
Diagana M, Preux PM, Dumas M. Diagana M, et al. J Neurol Sci. 2007 Nov 15;262(1-2):165-70. doi: 10.1016/j.jns.2007.06.041. Epub 2007 Jul 23. J Neurol Sci. 2007. PMID: 17643451 Review.
Cited by
- Detection of Binding Sites on SARS-CoV-2 Spike Protein Receptor-Binding Domain by Molecular Dynamics Simulations in Mixed Solvents.
Jokinen EM, Gopinath K, Kurkinen ST, Pentikainen OT. Jokinen EM, et al. IEEE/ACM Trans Comput Biol Bioinform. 2021 Jul-Aug;18(4):1281-1289. doi: 10.1109/TCBB.2021.3076259. Epub 2021 Aug 6. IEEE/ACM Trans Comput Biol Bioinform. 2021. PMID: 33914685 Free PMC article. - Recent advances in understanding Japanese encephalitis.
Banerjee A, Tripathi A. Banerjee A, et al. F1000Res. 2019 Nov 13;8:F1000 Faculty Rev-1915. doi: 10.12688/f1000research.19693.1. eCollection 2019. F1000Res. 2019. PMID: 31781366 Free PMC article. Review. - Japanese encephlu emergence in Australia: the potential population at risk.
Khan A, Riaz R, Nadeem A, Amir A, Siddiqui T, Batool UEA, Raufi N. Khan A, et al. Ann Med Surg (Lond). 2024 Jan 22;86(3):1540-1549. doi: 10.1097/MS9.0000000000001739. eCollection 2024 Mar. Ann Med Surg (Lond). 2024. PMID: 38463109 Free PMC article. Review. - In silico binding analysis of lutein and rosmarinic acid against envelope domain III protein of dengue virus.
Panchal R, Bapat S, Mukherjee S, Chowdhary A. Panchal R, et al. Indian J Pharmacol. 2021 Nov-Dec;53(6):471-479. doi: 10.4103/ijp.IJP_576_19. Indian J Pharmacol. 2021. PMID: 34975135 Free PMC article. - An Aloe-Based Composition Constituting Polysaccharides and Polyphenols Protected Mice against D-Galactose-Induced Immunosenescence.
Yimam M, Horm T, O'Neal A, Jiao P, Hong M, Jia Q. Yimam M, et al. J Immunol Res. 2024 Mar 14;2024:9307906. doi: 10.1155/2024/9307906. eCollection 2024. J Immunol Res. 2024. PMID: 38516617 Free PMC article.
References
- Chambers, T. J., T. F. Tsai, Y. Pervikov, and T. P. Monath. 1997. Vaccine development against dengue and Japanese encephalitis: report of a World Health Organization meeting. Vaccine 15:1494-1502. - PubMed
- Ghoshal, A., S. Das, S. Ghosh, M. K. Mishra, V. Sharma, P. Koli, E. Sen, and A. Basu. 2007. Proinflammatory mediators released by activated microglia induces neuronal death in Japanese encephalitis. Glia 55:483-496. - PubMed
- Kolson, D. L., E. Lavi, and F. Gonzalez-Scarano. 1998. The effects of human immunodeficiency virus in the central nervous system. Adv. Virus Res. 50:1-47. - PubMed
- Krady, J. K., A. Basu, C. M. Allen, Y. Xu, K. F. LaNoue, T. W. Gardner, and S. W. Levison. 2005. Minocycline reduces proinflammatory cytokine expression, microglial activation, and caspase-3 activation in a rodent model of diabetic retinopathy. Diabetes 54:1559-1565. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources