Psychological stress compromises CD8+ T cell control of latent herpes simplex virus type 1 infections - PubMed (original) (raw)
Psychological stress compromises CD8+ T cell control of latent herpes simplex virus type 1 infections
Michael L Freeman et al. J Immunol. 2007.
Abstract
Recurrent HSV-1 ocular disease results from reactivation of latent virus in trigeminal ganglia, often following immunosuppression or exposure to a variety of psychological or physical stressors. HSV-specific CD8+ T cells can block HSV-1 reactivation from latency in ex vivo trigeminal ganglia cultures through production of IFN-gamma. In this study, we establish that either CD8+ T cell depletion or exposure to restraint stress permit HSV-1 to transiently escape from latency in vivo. Restraint stress caused a reduction of TG-resident HSV-specific CD8+ T cells and a functional compromise of those cells that survive. Together, these effects of stress resulted in an approximate 65% reduction of cells capable of producing IFN-gamma in response to reactivating virus. Our findings demonstrate persistent in vivo regulation of latent HSV-1 by CD8+ T cells, and strongly support the concept that stress induces HSV-1 reactivation from latency at least in part by compromising CD8+ T cell surveillance of latently infected neurons.
Conflict of interest statement
Disclosures The authors have no financial conflict of interest.
Figures
FIGURE 1
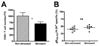
CD8 T cells are required to prevent viral DNA synthesis. Thirty days after HSV-1 corneal infection, mice harboring latent virus in their TG received a single i.p. injection of anti-CD8α mAb. Three days after treatment, TG were excised from CD8-depleted and nondepleted mice, and dispersed cells were analyzed by flow cytometry for their expression of CD8α and CD8β. A, Dot plots demonstrate a lack of CD8+ T cells in TG of anti-CD8α mAb-treated mice gated on CD45+ cells. B, DNA from ganglia of CD8-depleted mice (n = 5) contained a significantly (p < 0.009, Student’s t test) higher viral genome copy number than that from nondepleted mice (n = 20) when analyzed by quantitative real-time PCR for the HSV-1 glycoprotein H gene.
FIGURE 2
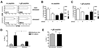
Restraint stress during latency increases HSV-1 genome copy number in the TG. A, Model of restraint stress protocol. B, Sera of nontreated mice or mice that were food and water deprived (FWD) but not stressed (nonstressed) contained significantly (p < 0.0001, one-way ANOVA and Tukey’s post test) less corticosterone (CORT) than sera from stressed mice when analyzed by a standard radioimmunoassay. C, TG were excised from stressed and nonstressed mice at the indicated time after infection and the HSV-1 genome copy number was determined by real-time PCR. **, p = 0.0039, Student’s t test. D, Frozen sections of TG obtained from stressed and nonstressed mice at 34 days after infection and stained for HSV-1 Ags. Arrows, Regions of positive staining for HSV-1 Ags.
FIGURE 3
Stress reduces CD8+ T cells in latently infected TG. Latently infected mice were subjected to restraint stress as described in Fig. 2. After the fourth restraint stress session (34 days after infection), TG were excised from stressed and nonstressed mice, dispersed into single-cell suspensions, and simultaneously stained with anti-CD8α mAb and tetramers containing the immunodominant HSV-1 gB498–505 epitope (gB498–505 H2-Kb). A, The mean absolute number of CD8+ T cells/TG (±SEM) in stressed (n = 23) and nonstressed (n = 16) mice. *, p = 0.03, Student’s t test. B, Percentage of CD8+ T cells that recognize the gB498–505 epitope. Group differences were not significant (p = 0.1436, Student’s t test).
FIGURE 4
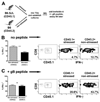
Stress compromises the function of HSV-specific CD8+ T cells in latently infected TG. Latently infected mice were subjected to restraint stress as described in Fig. 2. After the fourth restraint stress session (34 days after infection), TG were excised from stressed and nonstressed mice, dispersed into single-cell suspensions, and incubated for 72 h. TG cells were stained with CFSE and cultured with or without added HSV-1 gB498–505 peptide. After incubation, the cells were stained with anti-CD8α mAb and CFSE staining intensity of CD8+ T cells was analyzed by flow cytometry. A, Representative dot plots show extent of CFSE dilution in stressed and nonstressed cultures gated on CD45+ CD8+ cells. The box designates cells that have undergone one or more rounds of cell proliferation, and the MFI of these cells is shown. B and C, Data are presented as the mean (±SEM) percentage of CD8+ T cells that underwent one or more rounds of proliferation, and MFI of proliferating cells in cultures without peptide (B, n = 8/group) or with added peptide (C, n = 13–14/group). D, Single-cell suspensions of TG from stressed and nonstressed mice were incubated directly ex vivo for 6 h without stimulation in the presence of GolgiPlug (directly ex vivo, n = 3–4/group) or were cultured for 72 h and received GolgiPlug (TG culture, n = 13–17/group) for the last 6 h of incubation, stained for surface CD8 and intracellular IFN-γ, and analyzed by flow cytometry. Data are presented as the mean (±SEM) percentage of IFN-γ-positive CD8+ T cells. *, p < 0.05. E, Cultures received HSV-1 gB498–505 peptide (n = 4/group) and GolgiPlug for the last 6 h of the 72-h incubation, were stained for surface CD8α and intracellular IFN-γ, and analyzed by flow cytometry. Data are presented as the mean (±SEM) percentage of IFN-γ-positive CD8+ T cells. **, p < 0.01, all data were analyzed with Student’s t test.
FIGURE 5
CD8+ T cells in TG of stressed mice exhibit an intrinsic compromise of IFN-γ production. A, The preparation of mixed TG cultures from stressed (CD45.1) and nonstressed (CD45.2) latently infected congenic mice. Congenic mice were infected with HSV-1 and 30 days later were stressed or not, TG were obtained and dispersed into single-cell suspensions, and mixed cultures of TG from stressed and nonstressed mice were prepared as depicted. After 90 h, GolgiPlug was added to cultures without (B) or with (C) HSV-1 gB498–505 peptide for the last 6 h of incubation. Cells were recovered from cultures and surface stained with anti-CD8α and anti-CD45.1 mAb followed by intracellular stain for IFN-γ. Representative dot plots show recovery of CD8+ T cells that originated from stressed (CD45.1) and nonstressed (CD45.2) TG from the mixed cultures and IFN-γ expression in each population. Percents in lower right corner indicate the percentage of CD8+ T cells in the gated population. The graph shows the mean (±SEM) percentage of CD8+ T cells from stressed and nonstressed TG that expressed IFN-γ in the mixed TG cultures (n = 18). Data were analyzed with a Student’s paired t test.
Similar articles
- Influence of an immunodominant herpes simplex virus type 1 CD8+ T cell epitope on the target hierarchy and function of subdominant CD8+ T cells.
Treat BR, Bidula SM, Ramachandran S, St Leger AJ, Hendricks RL, Kinchington PR. Treat BR, et al. PLoS Pathog. 2017 Dec 4;13(12):e1006732. doi: 10.1371/journal.ppat.1006732. eCollection 2017 Dec. PLoS Pathog. 2017. PMID: 29206240 Free PMC article. - Delaying the expression of herpes simplex virus type 1 glycoprotein B (gB) to a true late gene alters neurovirulence and inhibits the gB-CD8+ T-cell response in the trigeminal ganglion.
Ramachandran S, Davoli KA, Yee MB, Hendricks RL, Kinchington PR. Ramachandran S, et al. J Virol. 2010 Sep;84(17):8811-20. doi: 10.1128/JVI.00496-10. Epub 2010 Jun 23. J Virol. 2010. PMID: 20573821 Free PMC article. - CD8+ T cells patrol HSV-1-infected trigeminal ganglia and prevent viral reactivation.
St Leger AJ, Hendricks RL. St Leger AJ, et al. J Neurovirol. 2011 Dec;17(6):528-34. doi: 10.1007/s13365-011-0062-1. Epub 2011 Dec 8. J Neurovirol. 2011. PMID: 22161682 Review. - Control of HSV-1 latency in human trigeminal ganglia--current overview.
Held K, Derfuss T. Held K, et al. J Neurovirol. 2011 Dec;17(6):518-27. doi: 10.1007/s13365-011-0063-0. Epub 2011 Dec 3. J Neurovirol. 2011. PMID: 22139603 Review.
Cited by
- Sensory Nerve Retraction and Sympathetic Nerve Innervation Contribute to Immunopathology of Murine Recurrent Herpes Stromal Keratitis.
Yun H, Yin XT, Stuart PM, St Leger AJ. Yun H, et al. Invest Ophthalmol Vis Sci. 2022 Feb 1;63(2):4. doi: 10.1167/iovs.63.2.4. Invest Ophthalmol Vis Sci. 2022. PMID: 35103749 Free PMC article. - Development and pathogenic evaluation of recombinant herpes simplex virus type 1 expressing two fluorescent reporter genes from different lytic promoters.
Ramachandran S, Knickelbein JE, Ferko C, Hendricks RL, Kinchington PR. Ramachandran S, et al. Virology. 2008 Sep 1;378(2):254-64. doi: 10.1016/j.virol.2008.05.034. Epub 2008 Jul 11. Virology. 2008. PMID: 18619637 Free PMC article. - Adiponectin Receptors Are Less Sensitive to Stress in a Transgenic Mouse Model of Alzheimer's Disease.
Várhelyi ZP, Kálmán J, Oláh Z, Ivitz EV, Fodor EK, Sántha M, Datki ZL, Pákáski M. Várhelyi ZP, et al. Front Neurosci. 2017 Apr 11;11:199. doi: 10.3389/fnins.2017.00199. eCollection 2017. Front Neurosci. 2017. PMID: 28442988 Free PMC article. - Influence of an immunodominant herpes simplex virus type 1 CD8+ T cell epitope on the target hierarchy and function of subdominant CD8+ T cells.
Treat BR, Bidula SM, Ramachandran S, St Leger AJ, Hendricks RL, Kinchington PR. Treat BR, et al. PLoS Pathog. 2017 Dec 4;13(12):e1006732. doi: 10.1371/journal.ppat.1006732. eCollection 2017 Dec. PLoS Pathog. 2017. PMID: 29206240 Free PMC article. - Mucosal immunology of the ocular surface.
de Paiva CS, St Leger AJ, Caspi RR. de Paiva CS, et al. Mucosal Immunol. 2022 Jun;15(6):1143-1157. doi: 10.1038/s41385-022-00551-6. Epub 2022 Aug 24. Mucosal Immunol. 2022. PMID: 36002743 Free PMC article. Review.
References
- Padgett DA, Glaser R. How stress influences the immune response. Trends Immunol. 2003;24:444–448. - PubMed
- Sainz B, Loutsch JM, Marquart ME, Hill JM. Stress-associated immunomodulation and herpes simplex virus infections. Med. Hypotheses. 2001;56:348–356. - PubMed
- Shimeld C, Whiteland JL, Williams NA, Easty DL, Hill TJ. Cytokine production in the nervous system of mice during acute and latent infection with herpes simplex virus type 1. J. Gen. Virol. 1997;78:3317–3325. - PubMed
- Halford WP, Gebhardt BM, Carr DJ. Persistent cytokine expression in trigeminal ganglion latently infected with herpes simplex virus type 1. J. Immunol. 1996;157:3542–3549. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 AI060525/AI/NIAID NIH HHS/United States
- P30 EY 08098/EY/NEI NIH HHS/United States
- R01 EY005945/EY/NEI NIH HHS/United States
- P30 EY008098-19/EY/NEI NIH HHS/United States
- R01 AI049719/AI/NIAID NIH HHS/United States
- P30 EY008098-20/EY/NEI NIH HHS/United States
- AI 49719/AI/NIAID NIH HHS/United States
- EY 05945/EY/NEI NIH HHS/United States
- R01 EY005945-21/EY/NEI NIH HHS/United States
- T32 AI 060525/AI/NIAID NIH HHS/United States
- T32 AI060525-01A1/AI/NIAID NIH HHS/United States
- R01 EY005945-22/EY/NEI NIH HHS/United States
- 5T32 AI 049820/AI/NIAID NIH HHS/United States
- T32 AI049820/AI/NIAID NIH HHS/United States
- P30 EY008098/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous